Cultivate Compliance
Harmonize and streamline regulatory compliance
Created in Canada to help licensed producers meet Canadian and international regulatory compliance, AirMed also provides quality management tools to minimize risks, increase productivity, and enhance customer satisfaction.
Your Compliance
AirMed was designed to meet all quality standards for record-keeping systems, which means that AirMed is not only GPP-ready but also GMP-ready and GACP-ready. GMP-certification is required to export directly to many regions, but becoming certified can be expensive & time-consuming. GACP-certification can be faster & more economical, as it does not require equipment & software validation. To take advantage of overseas markets, GACP-certified LPs can sell bulk product to GMP-certified distributors.




Good ProductionPractice
Good ManufacturingPractice
Good Agricultural& Collection Practice
Current GoodManufacturing Practice
Standard designed for cannabis in Canada, which combines guidelines from Good Manufacturing Practice & Good Agricultural Practice
Standard in the European Union & other regions including Australia for pharmaceuticals, therapeutic goods & herbal supplements
Standard for agricultural products around the world, which is often applied to the cultivation & distribution of cannabis
The US Food & Drug Administration standard for pharmaceuticals & herbal supplements, which includes cannabis
Computerized System
AirMed meets the computerized systems guidelines for quality standards including Health Canada and EU GMP as well as general business standards such as ISO.
Privacy & Security
Hosted in a 3rd party, SSAE 16 SOC 2 Type 2-certified data centre in Canada that complies with Canadian Privacy and Security regulations as well as HIPAA and PCI; multi-layered protection including network, hypervisor and virtual machine layer security features
Encryption & Integrity
Sensitive data is encrypted at rest and in transit; information is secured by both physical and electronic means against damage; stored data is checked for accessibility, readability and accuracy; access to data is ensured throughout the retention period
Audit Logs & Backups
Complete audit trails record every action; administrators can review changes anywhere at any time; full backups with fully redundant architecture using enterprise VMware load-balanced servers running an enterprise class database
Access Control
Administrators configure precise access based on worker security profiles; electronic signatures and multi-factor authentication ensures only authorized users access data and complete tasks.
Implementation
As a cloud-based or software-as-a-service (SaaS) application, no hardware, installation, or maintenance are needed; a help system provides product documentation and a shared database lets authorized users practice working with the software
Auto Updates
As new regulations are introduced, AirMed is updated automatically without the need for customers to apply updates or make additional customizations
AirMed Compliance Highlights
Designed to meet or exceed all Canadian regulations as well as international guidelines, AirMed is a better fit for your cannabis business than agro or greenhouse software. Processes with compliance implications are streamlined into easy repeatable workflows with full audit trails and automated reporting.

Whether starting with seeds, clones or tissue culture, AirMed tracks all information about the growth cycle including environmental conditions, nutrients, and experimental criteria.
Our system also tracks receipt of new source material, propagation, the vegetative cycle, flowering cycle, harvesting, packaging, sample management, waste management, destruction events, and the preparation of facilities for each grow cycle.
Support for GS-1 barcoding eases the complexity of production, packaging, quality-assurance, inventory management, and sale & distribution.
From the moment genetics enter your facility until the product arrives at its destination, AirMed helps you manage every stage. All workflows are integrated into a single secure Cloud application ensuring data integrity and quality control while simplifying compliance.

AirMed provides a comprehensive suite of cannabis management and record-keeping features to maintain and record all activity and compliance, including quality assurance and relevant SOPs.
Track all cannabis material from receiving, through the production cycles, packaging, testing, and purchase order through fulfillment and shipping to clients or wholesale purchasers.
Use robust communication management tools with secure privacy features to record and track every interaction/communication with authorities, suppliers & clients.
AirMed enforces checks on key safety regulations. Electronic records backed by generated printable reports/forms ensure easy Cannabis Act compliance.

AirMed tracks and logs every employee and client action to ensure audit-readiness, simplifying compliance for producers and regulatory bodies with reports and analytics.
Complete audit trails for every record and every action let administrators review who browsed, edited, added or deleted data anywhere in the system at any time.
Administrators can configure precise access based on an employee security profile, and electronic signatures and multi-factor authentication ensure only authorized employees can access data and complete tasks.
Detailed audit reports provide specifics on plants, destruction events, sample management, weights, and complete product traceability.

AirMed software offers full functionality for multi-level packaging with layered barcoding that meets GS-1 barcode standards. This is the standard required to sell to provincial governments and to many other distributors worldwide.
Licensed producers who use AirMed can assemble retail-ready individual product packages using a ‘master case’ system and identify and track products at each packaging level.
The AirMed track-and-trace system allows retail packages to be traced to the lot or batch and ultimately back to the original genetic material. And AirMed has a comprehensive multi-step recall process that completely automates recalls if they are ever needed.

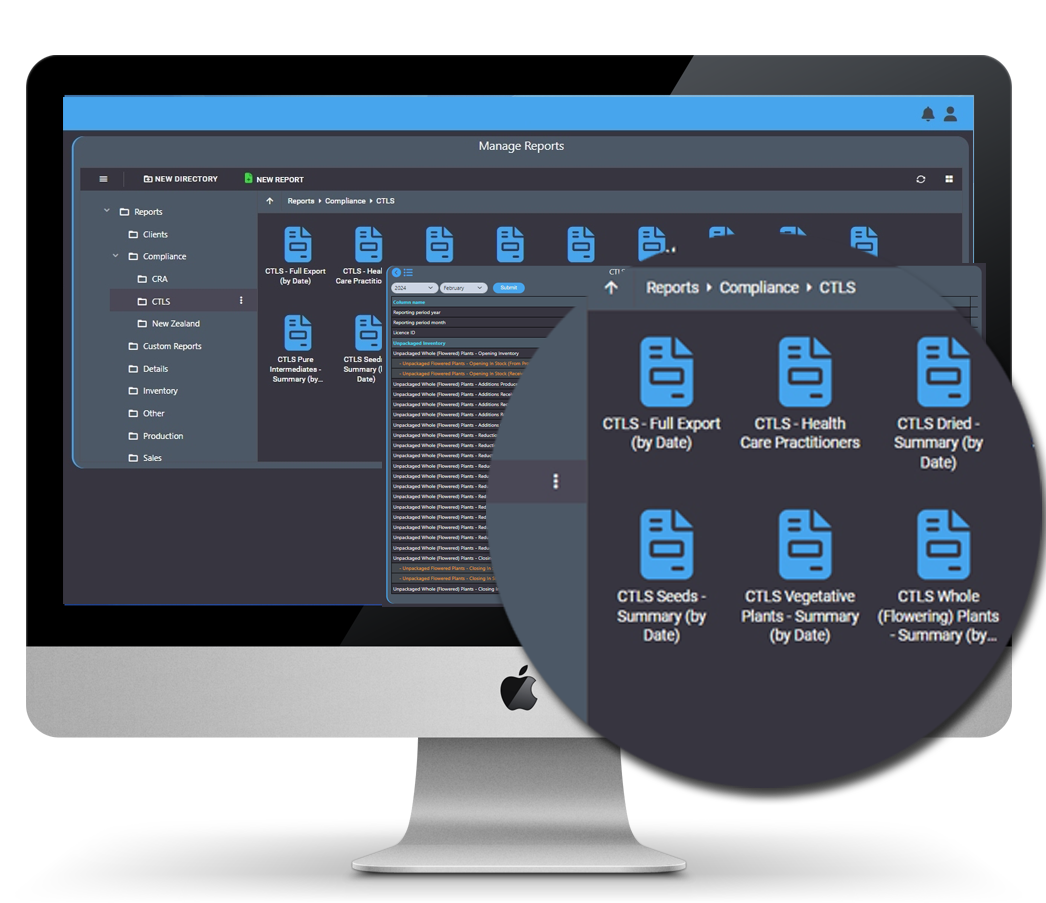
Always innovating to help our customers meet the regulatory challenges they face daily, AirMed offers an automated system for Health Canada CTLS reporting.
Full Export: Using our full export process, thousands of reported fields tracked by AirMed automatically populate the Health Canada template. Not only is the time required to complete monthly reports reduced, but the potential for human error often encountered in manual data entry is eliminated.
Summary Reports: Our CTLS summary reports breakdown key fields with links to details that identify the exact inventory records and values used to calculate totals.
Our Compliance
A software system cannot become GMP-certified. Only a manufacturing facility can achieve that qualification. The role of software in cannabis compliance is to deliver the means for managing & tracking regulated processes in a production or manufacturing facility and to provide record-keeping for auditing. Our responsibility as a vendor is to ensure that our software meets the specified guidelines and can be validated against your practices.

Vendor Validation
Our organization successfully passed an external audit by a pharmaceutical manufacturer to determine if the organization was suitable as a supplier and if the company's software was acceptable for use in the regulated environment.

ISO Review
Our organization underwent a review by a certified ISO consultant to ensure that internal policies, procedures & documentation met the guidelines of quality systems including those of the International Organization for Standardization.
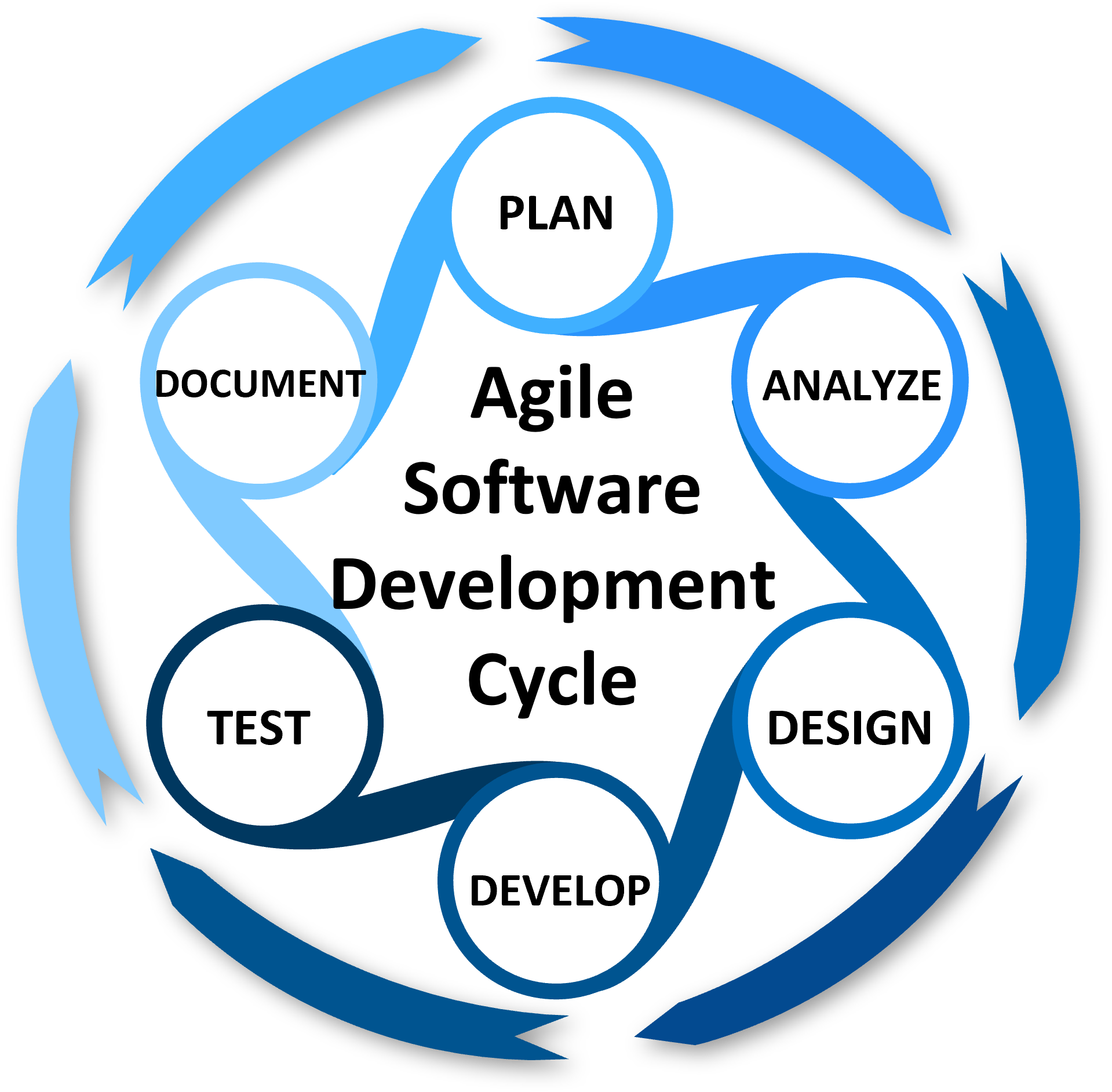
Software Development
AirMed conforms to an acceptable Software Development Life Cycle (SDLC) and follows standard operating procedures for all critical processes related to design, testing, product release and maintenance.
