Cannabis & GMP/GACP: Part 2 - Quality Systems

Welcome to our summer series on cannabis and GMP/GACP. A new article will be published once a week throughout the summer. You can access related articles that have been published so far by clicking the Compliance category on the main News & Events index page: Compliance category
Cannabis Around the World
The legality of cannabis varies from around the world. In some regions, cannabis is licensed for both medical and recreational use. In others, cannabis is licensed for medical use only. And in some, cannabis remains a prohibited substance for any use.
At the time of writing this, 50 nations had legalized cannabis nationally or regionally for medical or recreational use. Each individual region sets the standards for cannabis production, manufacturing and distribution.
The types of standards that are applied to cannabis are collectively known as ‘quality systems.’ Most quality systems are similar to each other, using accepted good practices. What differs are the processes they cover, which are dependent on the products being manufactured.
In many cases, the standards applied to cannabis are based on pre-existing quality systems designed for other types of manufacturing or production. Since the plants are grown in an agricultural setting, agricultural standards, such a Good Agricultural & Collection Practice (GACP), can be applied. As cannabis has drug qualities and therapeutic value, pharmaceutical standards, such as Good Manufacturing Practice (GMP), can be applied. With testing for THC and other values, clinical or laboratory standards can be applied. As well, general business standards, such as those from the International Organization for Standardization (ISO), can also be applied.
New standards, however, can be created at any time. When Canada was legalizing cannabis, Health Canada created a new quality system designed specifically for cannabis called Good Production Practice (GPP).
When other regions were considering cannabis, however, most decided not to create a new standard as Canada did and chose GMP or GACP instead.
Each of these quality systems has the same type of framework. Rather than rigid rules, most standards provide guidelines for carrying out operations. The standards are not designed to limit how a business operates. Instead, they are intended to guide the facility toward good practices. The ‘how’ of complying is left up to the individual organization. Each facility is allowed to determine the best way to meet the criteria. The final result is what is important.
Quality Standards Benefits
Besides meeting compliance regulations, adhering to a quality system offers many benefits, including increased productivity, improved employee safety, and enhanced customer satisfaction. But one of the main purposes of standards in relation to cannabis is to minimize the following risks.
- Contamination of products that could make them unsafe.
- Inaccurate labelling that could lead to accidental misuse.
- Insufficient active ingredients that could affect efficacy.
- Excess active ingredients that could be hazardous.
These risks are the reason cannabis operations must comply with a quality system, regardless of where they are in the world. There may be one governing body with one set of standards clearly laid out. In other cases, there may be several sets of standards that focus on different areas of operation. When setting up a facility, each cannabis business must identify which standards will apply and how to meet them.
Links
Following are links to the various standards that could be applied to cannabis.
Good Agricultural & Collection Practices/GACP (also called GAP): International; Israel
https://www.who.int/publications/i/item/9241546271
https://www.gov.il/en/departments/topics/medical-cannabis/govil-landing-page
Good Production Practices GPP: Canada
Good Manufacturing Practices/GMP: International; European Union; Australia
Current Good Production Practices/cGMP: USA
Good Distribution Practices/GDP: United Kingdom
https://www.gov.uk/guidance/good-manufacturing-practice-and-good-distribution-practice
Cannabis & GMP/GACP: Part 1 – The Compliance Ecosystem

Welcome to our summer series on cannabis and GMP/GACP. A new article will be published once a week throughout the summer. You can access related articles that have been published so far by clicking the Compliance category on the main News & Events index page: Compliance category
Cannabis as a High Consequence Industry
High consequence is a term that has been used to describe those industries where compliance is essential. High consequence equals highly regulated, and for good reason. As the name suggests, errors in the activities of these organizations have severe consequences. Typically, these are industries where human life or quality of life is at stake, but not always. High consequence might relate to financial or intellectual property security. Examples of high consequence industries include food production, biotech, healthcare, aviation, nuclear power, and law enforcement. Due to the legal and health implications, the production and sale of cannabis is a high-consequence industry.
But even for organizations in low or medium consequence industries, regulations still apply. No one in today’s world operates without rules. In fact, the current business environment is an increasingly complex maze of regulations.
Legislation and standards are in place or being proposed for almost all aspects of business and industry. Throughout the world, regulations govern the manufacturing and handling of a variety of products for health and safety reasons. Other policies are designed to provide privacy and security or prevent fraud. These regulations are administered by government agencies, international organizations and industry associations, and compliance is sometimes voluntary but often mandatory.
Record Management
Many of these laws and guidelines focus on the maintenance, security and auditing of records.
The International Organization for Standardization (ISO) defines Records Management as “the field of management responsible for the efficient and systematic control of the creation, receipt, maintenance, use and disposition of records, including the processes for capturing and maintaining evidence of and information about business activities and transactions in the form of records.” Electronic records management is the digital method for doing this.
Electronic records are critical to doing business today, especially in high-consequence industries where auditing and reporting are required to meet compliance.
Of course, the electronic records themselves are only part of what is necessary for compliance with legislation or standards. The internal policies and procedures that surround those electronic records form an important piece of the compliance puzzle.
Since a computer record is only as secure as the system that holds it, the organization must therefore make certain that the hardware and software comprising that system is secure as well. And since the hardware and software are only as secure as the facility they are located in, this also means securing the physical location of any servers or back-up copies of data and so on. No electronic record or system can meet such requirements—only an internal security policy and its associated procedures can.
While compliance with regulations is dependent on internal policies and procedures, computer systems and the software are often the means of carrying out those policies and procedures. An electronic records management system must therefore offer certain functionality to authenticate and validate the integrity of its records.
The policies of most regulatory bodies include language directed at ensuring the security of electronic records and the systems that support them. These systems must let organizations create, implement and verify security and auditing related to regulatory compliance.
Regulated organizations are ultimately responsible for the computer systems in their facilities and for ensuring that those systems meet any regulations set out for them. However, since these organizations seldom design the systems they use, it falls to hardware and software vendors to interpret the standards and ensure that appropriate features and functions are available to meet them. This is where we come in.
Links
Following are links to the various standards that could be applied to cannabis.
Good Agricultural & Collection Practices/GACP (also called GAP): International; Israel
https://www.who.int/publications/i/item/9241546271
https://www.gov.il/en/departments/topics/medical-cannabis/govil-landing-page
Good Production Practices GPP: Canada
Good Manufacturing Practices/GMP: International; European Union; Australia
Current Good Production Practices/cGMP: USA
Good Distribution Practices/GDP: United Kingdom
https://www.gov.uk/guidance/good-manufacturing-practice-and-good-distribution-practice
RSMj 2024 Annual Scientific Meeting: July 19-21 in Toronto

The 8th Annual Scientific Meeting of the Research Society on Marijuana (RSMj) will be held from July 19-21, 2024 at the Downtown Toronto Hilton in Toronto, Ontario, Canada. This year’s meeting is in collaboration with the Michael G. DeGroote Centre for Medicinal Cannabis Research, a joint research centre of McMaster University and St. Joseph’s Healthcare Hamilton.
The Research Society on Marijuana (RSMj) is a network of scientists with the shared goal of promoting understanding through empirical research of the determinants, correlates, consequences, contexts, and assessment of marijuana use as well as the treatment of problematic marijuana use, including cannabis use disorder
Virtual CME/CE options are available for those who are only interested in attending virtually as part of a separate program.
For more information visit:
https://www.researchmj.org/meeting
AirMed Supports Direct Delivery
What is Direct Delivery?
Direct delivery programs let licensed producers sell their products directly to retailers, which can eliminate warehousing. In British Columbia, the standard practice is for LPs to send cannabis to the BC Liquor Distribution Branch’s (LDB) warehouse. Dispensaries must then choose the products they wish to buy from the LDB product list.
For retailers, the disadvantages include limiting choices to what’s available in the warehouse and typically requiring a minimum order amount. This process also does not allow retailers to select products based on freshness.
For LPs, there is no assurance that the cannabis sent to the warehouse will be ordered within its shelf life. Yet each time a product is shipped, the production facility must use a pre-paid excise stamp. As a result, LPs spend the price of the excise stamp without a guarantee of sale. The possibility exists for the cost of producing and shipping the product to be thrown away, plus there’s the extra work involved in reclaiming the excise tax.
Direct delivery lets LPs register and list products for sale through a provincial direct-to-retailer program. Dispensaries can choose products from an individual LP and arrange for delivery directly from production facility to storefront. The shipment still requires an excise stamp and provincial fees, but the sale to the retailer has already been made, which covers those costs.
The benefits of direct delivery include:
- Giving retailers greater choice when ordering plus no minimum sales and potentially shorter delivery times
- Supplying dispensaries and consumers with fresher product
- Letting LPs cultivate-to-order and preventing products from expiring in warehouses
- Removing speculative costs of excise stamps for LPs and ensuring up-front revenue to LPs
- Helping small-scale producers be more competitive in the marketplace by creating opportunities for brand marketing and promoting better customer service to retailers
How AirMed Supports Direct Delivery
AirMed Direct Delivery offers a streamlined service that enables licensed retail stores to bypass provincial warehouses and order products directly from licensed producers who use AirMed in provinces where this is permitted, currently British Columbia and Saskatchewan.
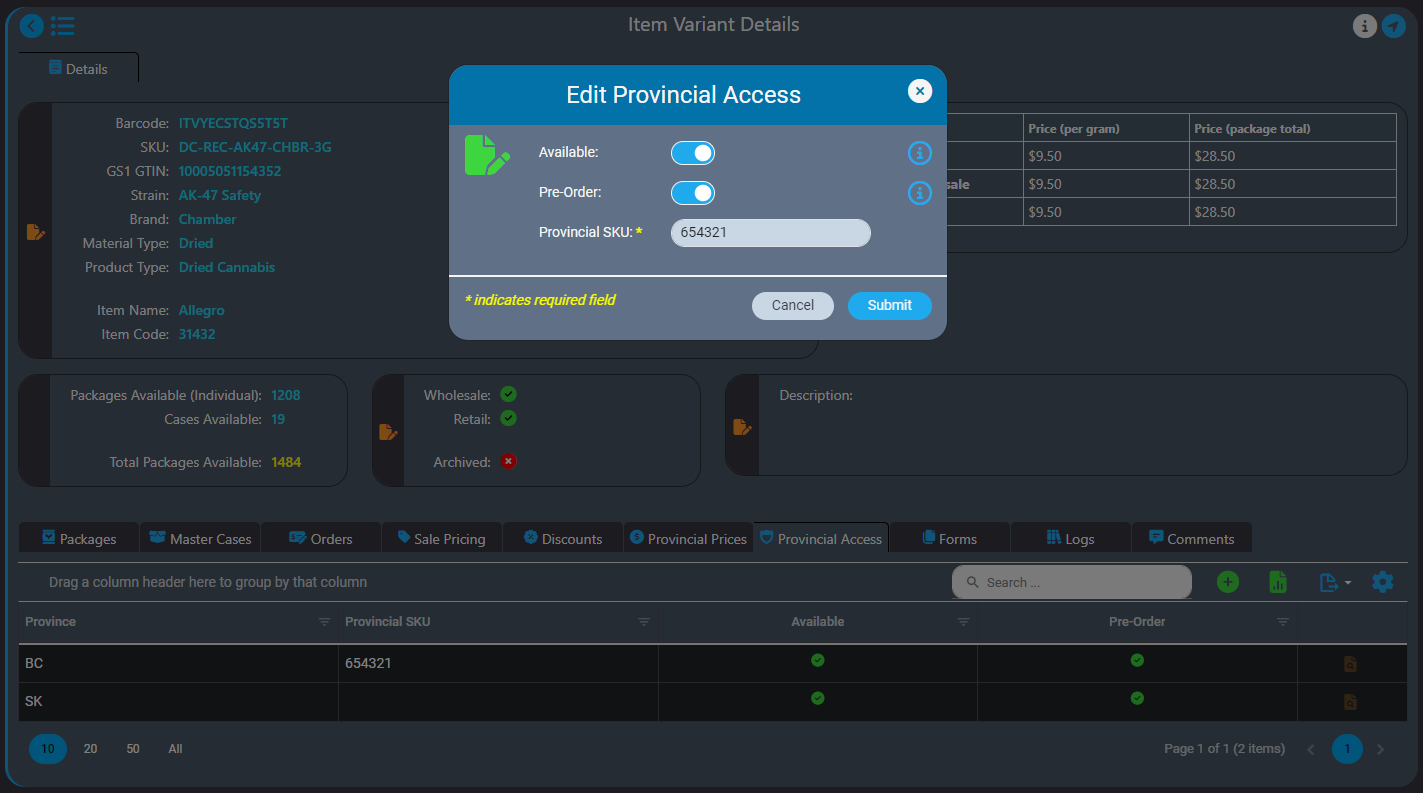
Our built-in workflows that support direct-delivery simplify your processes making it easier for you to work with your retail partners.
WordPress Plugin for Retailer Registration
A proprietary WordPress plugin and API provide fast and easy integration of your AirMed database with an online store on your website. This lets you offer your products directly to licensed retail outlets complete with a catalog and shopping cart. Retailers can register and get approval from licensed producers through the WordPress plugin. Approved retailers gain access to a product catalogue and order cases of finished packages.
Pricing and Orders
AirMed supports different pricing structures for various provinces for both individual packages and bulk cases. AirMed also allows pre-ordering of out-of-stock products, enabling producers to manufacture based on incoming orders.
Order Fulfillment
Once an order is placed, it is automatically processed in AirMed. Producers can fulfill orders, manage payments, and ship products directly to retail stores.
Provincial Support
Direct delivery is designed to comply with current provincial regulations and can adapt to include new provinces as regulations change. You can even set up direct-delivery product availability for provinces that may elect to allow direct delivery in the future.
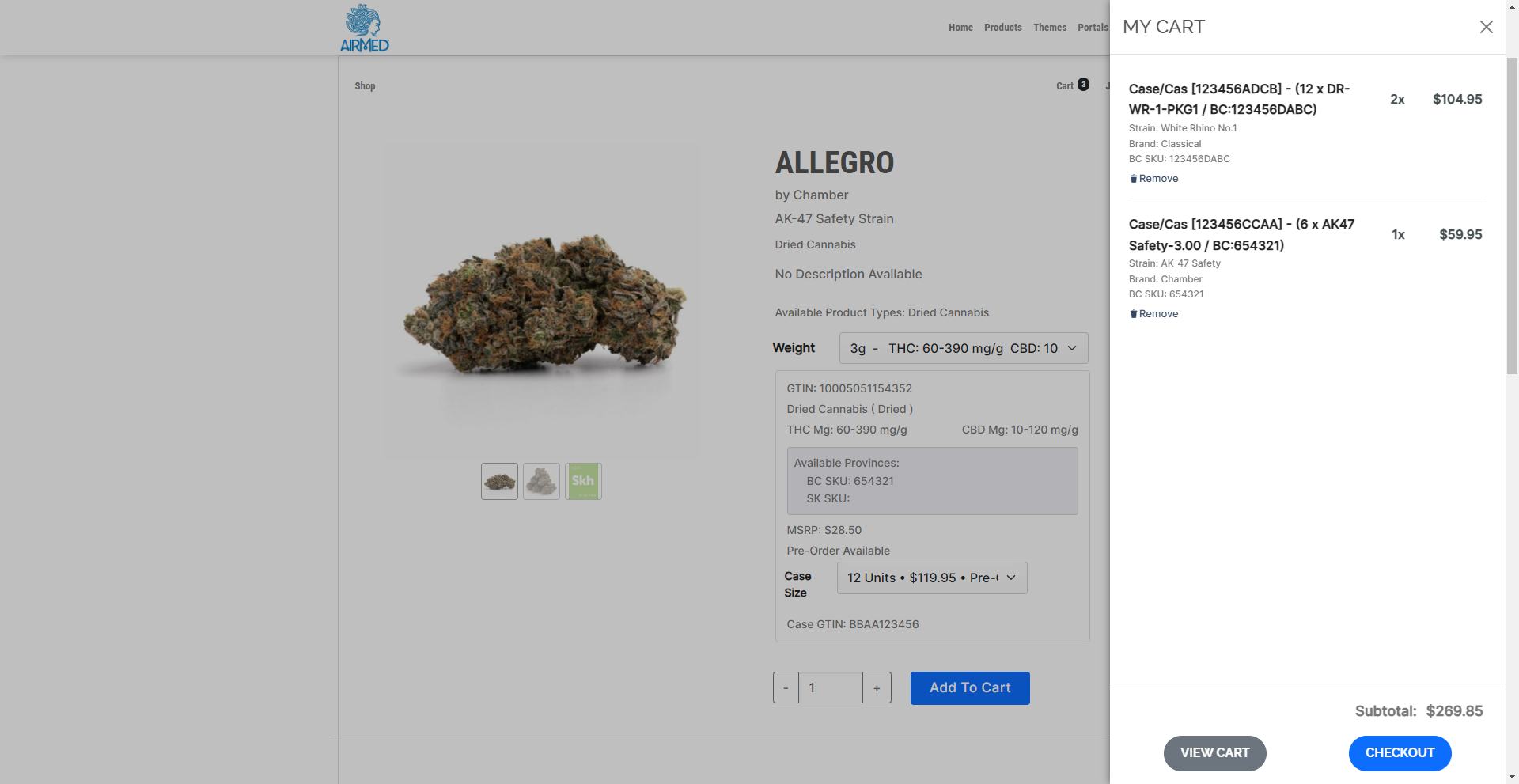
Our solution is ideal for those looking to streamline their supply chain and enhance their ordering process in the cannabis retail industry.
Efficiency: Retail stores can directly access products from producers who use AirMed, reducing the dependency on provincial warehouses and potentially shortening delivery times.
Flexibility: Producers can manage their inventory and production schedules more effectively through pre-orders, and AirMed manages creating product cases in varying sizes with associated case pricing for each product type and province.
Scalability: Our system can expand to include additional provinces as they permit direct delivery.
To see all the ways AirMed supports direct delivery, book a free demo by using the Request Demo button at the top of the page.
For more information on direct delivery in British Columbia, visit: https://www.bcldbcannabisupdates.com/LDBDirectDeliveryProgram
For more information on cannabis laws in Saskatchewan, visit: https://www.slga.com/cannabis
To read an article about direct delivery on the Stratcann website visit: https://stratcann.com/insight/bcs-
annabis-direct-delivery-program-is-growing-but-fees-still-too-high/c
Happy Canada Day!

We’d like to wish all our clients and fellow industry members a safe and happy Canada Day.
We hope we can all celebrate the beauty of our landscape, the warmth of our people, and the values of diversity and inclusivity that make us Canadians. And because the goodwill of those we serve is the foundation of our success, we’d also like to say thank you.
At this time, we’d also like to acknowledge and respect the Lekwungen-speaking Peoples on whose traditional territories we live and the Songhees, Esquimalt and WSANEC Peoples whose historical relationships with the land continue to this day.
For more information visit our Software page.
Celebrating the 50th anniversary of the barcode

The barcode, originally based on Morse Code, was invented and patented in the USA by Norman Joseph Woodland and Bernard Silver in 1952. However, it was more than 20 years before the invention became commercially successful.
Various organizations and companies played a role in the use of the barcode during those 20 years, but the real success began with a meeting of industry leaders in New York city in March 1971. It took another three years before a barcode that could be both printed easily and scanned easily was finally developed. In April of 1973, an industry-wide agreement on that barcode became the basis for GS1.
GS1, which stands for Global Standards, is a not-for-profit international organization that develops and maintains standards for barcodes and packaging identification.
The first barcode was scanned in the USA on the 26th of June 1974. Canada was the second country to introduce the barcode when a grocery store in Dorval, Quebec scanned the very first Canadian barcode.
The barcode was designed to make it easier for products to be tracked, processed, and stored. Today, these barcodes play a critical part in supply chains across the globe.
Available from 116 GS1 offices around the world, GTIN or Global Trade Item Number provides unique codes for a company and its products that can identified using electronic scanners.
GTIN employs a multi-digit number that is primarily used within barcodes. In North American, the UPC (Universal Product Code) is an existing form of the GTIN. In Europe, EAN-13 is the standard.
Cheers to all who had a hand in creating this useful tool that revolutionized the retail industry 50 years ago today.
For more information on GS1 and GTIN, visit,
https://www.gs1.org/standards/barcodesFor a history of the barcode, visit:
https://en.wikipedia.org/wiki/Barcode
Indigenous History Month and Cannabis News

AirMed would like to acknowledge June as National Indigenous History Month in Canada by providing links to Indigenous news and resources related to our industry.
APTN News recently discussed the 2024 federal budget with its cannabis implications for Indigenous communities in a feature on its website. Read it here: https://www.aptnnews.ca/featured/budget-2024-ottawa-federal-government-ontario-money-first-nations-inuit-metis/
Stratcann, the Canadian cannabis news and events platform for industry professionals, published a feature entitled, “Indigenous History Month focus: Indigenous cannabis news.” The post was a review of StratCann news “spotlighting Indigenous voices in both the successes and disputes, which are being heard loudly and clearly. Regardless of the outcome, the recognition of Indigenous peoples in the Canadian cannabis industry is stronger than ever.” Read it here: https://stratcann.com/news/indigenous-history-month-focus-indigenous-cannabis-news/
The Canadian Bar Association of British Columbia published a post recently titled, “Respecting Indigenous Regulation of Cannabis.” With the subtitle, “Time to turn over a new leaf,” the article discusses jurisdictional issues related to cannabis in Canada. Read it here: https://www.cbabc.org/BarTalk/Articles/2024/April/Features/Respecting-Indigenous-Regulation-of-Cannabis
In related news, a Nova Scotia judge rejected constitutional arguments for Indigenous cannabis shops. CityNews in Halifax posted a story about it here: https://halifax.citynews.ca/2024/06/14/nova-scotia-judge-rejects-constitutional-arguments-for-indigenous-cannabis-shops/
CBC also discussed the issue here:
https://www.cbc.ca/news/canada/nova-scotia/cannabis-emerging-new-battleground-over-mikmaw-rights-1.7151120
Last year MJBiz Daily published “Canadian panel urges bigger role for First Nations in cannabis industry.” This article recapped a Canadian Senate committee’s recommendations for regulations related to cannabis for First Nations. Read it here: https://mjbizdaily.com/canadian-panel-urges-bigger-role-for-first-nations-in-cannabis-industry/
The First Nations Health Authority offers a variety of resources on its website designed to help Indigenous youth make informed choices by providing information on the health impacts of using cannabis. View the resources here: https://www.fnha.ca/what-we-do/mental-wellness-and-substance-use/non-medical-cannabis
Thanks for making Growing Relationships Kelowna a success!

As a sponsor, we’d like to thank everyone who made Growing Relationships Kelowna a success.
Hosted by StratCann, your premier cannabis news and events platform for industry professionals, this one-day event was held Monday June 10, 2024 at the Eldorado Manteo Resort.
Growing Relationships brought together British Columbia’s community of small-batch and micro producers, retailers and other cannabis professionals.
StratCann is capturing the notes and providing a summary to the relevant regulators to share what is happening with those who have their feet on the ground.
We hope you enjoyed this event as much as we did. Thanks again to attendees, vendors and organizers for a great day in Kelowna.
For more information visit: https://stratcann.com/event/growing-relationships-kelowna-2024/
Growing Relationships Kelowna is next Monday: Jun 10, 2024

We’re just days away from StratCann’s Growing Relationships in Kelowna.
Designed to deliver opportunities to build relationships and socialize with the community, StratCann brings retailers and producers together for a day of networking, brainstorming, discussion and learning.
This event features “Navigating the Green Frontier: AirMed’s Revolutionary Impact on Cannabis Management,” a presentation by Justin Hearn, president & CEO of AirMed.
StratCann delivers timely and relevant news, insights and events for cannabis industry professionals in Canada and around the world, and AirMed is proud to be a sponsor of this event.
Attendees will enjoy networking opportunities, engaging panel discussions, a delicious catered lunch, and access to the beautiful waterfront patio at the Eldorado Manteo Resort. We hope to see you there!
For more information visit: https://stratcann.com/growing-relationships/
Selling internationally? You need GS1 and GTIN!

GTIN or Global Trade Item Number is a standard from the GS1 (Global Standards) organization. GTIN consists of unique codes that identify manufacturers and their products using barcodes. When scanned by an electronic reader, the GTIN barcode provides a code that is related to a specific manufacturer and a specific product from that manufacturer.
In North American, the UPC (Universal Product Code) is an existing form of the GTIN. In Europe, EAN-13 is the GTIN standard.
The GTIN system lets you and your products be identified across the globe. If you hope to sell in certain parts of the world, such as Europe, you will need to use GS1 standards and GTIN codes. Once you have signed up with your regional GS1 office, you will be issued a series of unique codes to use on your product packaging.
Fully supporting GS1, AirMed prints your GTIN barcodes directly from the database. Our master case processing lets you use multi-level barcoding for several layers of packaging or stock-keeping units (SKU). For example, one case could contain a dozen smaller cartons. Each of those cartons could contain a dozen retail-ready individual packages. All of those packaging layers can have its own barcode to meet the retail standards of the region where it will eventually be sold.
AirMed helps you meet your barcoding and packaging standards whether selling to a provincial warehouse or shipping internationally. AirMed even lets you apply pricing at the package or the master case SKU, and custom pricing can be set for specific SKUs by customer.
For more information on GS1 standards and GTIN barcoding, visit: https://gs1ca.org/
If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or click the Request Demo button at the top of the page to start the ball rolling.
Cannabis Europa 2024: Jun 25-26 in London

The Cannabis Europa conference & expo is being held at The Barbican in London this June.
Cannabis Europa is proud to consistently deliver the highest quality content – presented by leading authorities in their field – tackling the topics and themes that are pivotal to the global growth of the cannabis industry.
Join 1,500+ policy makers, entrepreneurs, innovators and investors to shape the future of the maturing European cannabis market — a market that is set to be worth £3.2 billion by 2025.
Over 50 exhibitors and 1200 visitors are expected at next year’s Cannabis Europa expo.
For more information visit: https://www.cannabis-europa.com/
AirMed to Sponsor Growing Relationships Kelowna: Jun 10, 2024

We are excited to announce that AirMed is a proud sponsor of StratCann’s Growing Relationships event in Kelowna on June 10!
This event is designed to provide a platform for building relationships and engaging with the community through networking, brainstorming, discussions, and learning sessions.
Justin Hearn, President & CEO of AirMed, will be among the featured speakers, presenting “Navigating the Green Frontier: AirMed’s Revolutionary Impact on Cannabis Management.”
StratCann is dedicated to bringing together growers, processors, and retailers to foster connections, share insights, and discuss industry challenges.
Attendees can look forward to networking opportunities, informative panel discussions, a catered lunch, and access to the beautiful waterfront patio at the Eldorado Manteo Resort.
After the success of our sponsorship at the Calgary event, we are excited to support Growing Relationships once again in Kelowna. We would love to see you there!
We look forward to connecting with you at Growing Relationships Kelowna!
For more information visit: https://stratcann.com/growing-relationships/
European Cannabis Report 2024

The 9th Edition of the longest-standing annual report dedicated to Europe’s evolving and dynamic legal cannabis market is now available.
The European Cannabis Report: 9th Edition offers the most up-to-date insights and analysis on the evolution of key cannabis markets across Europe, including: Germany, the UK, Switzerland, the Netherlands and the Czech Republic.
Published by Prohibition Partners, a free-to-download version of the report is available that excludes market sizing analysis. For market sizing data and analysis, please see the paid-for packages.
For more information, including how to download a free copy of the report, visit:
https://prohibitionpartners.com/reports/the-european-cannabis-report-9th-edition/
Thanks for making Growing Relationships Calgary a success!

As a sponsor, we’d like to thank everyone who made Growing Relationships Calgary a success.
Hosted by Stratcann, your premier cannabis news and events platform for industry professionals, this one-day event was held Monday May 6, 2024 at the Calgary Marriott Hotel.
Growing Relationships brought together Alberta’s community of small-batch and micro producers, retailers and other cannabis professionals.
StratCann is capturing the notes and providing a summary to the relevant regulators to share what is happening with those who have their feet on the ground.
We hope you enjoyed this event as much as we did. Thanks again to attendees, vendors and organizers for a great day in Calgary.
For more information visit: https://stratcann.com/growing-relationships/
Only Four Days until Growing Relationships Calgary: May 6, 2024

We’re on our way to Calgary for Growing Relationships next Monday.
Stratcann is bringing this one-day event back to Alberta’s community of small-batch and micro producers, retailers and other professionals in the cannabis industry.
Specifically designed to deliver opportunities to build relationships and socialize with the community, this event brings retailers and producers together for a day of networking, brainstorming, discussion and learning.
AirMed is a proud sponsor of this industry event where attendees will enjoy networking opportunities, engaging panel discussions, a delicious catered lunch, and exclusive access to the Sunalta patio at the Calgary Marriott Downtown Hotel.
We hope to see you there!
For more information visit: https://stratcann.com/growing-relationships/
Data collection made easy with AirMed e-form designer
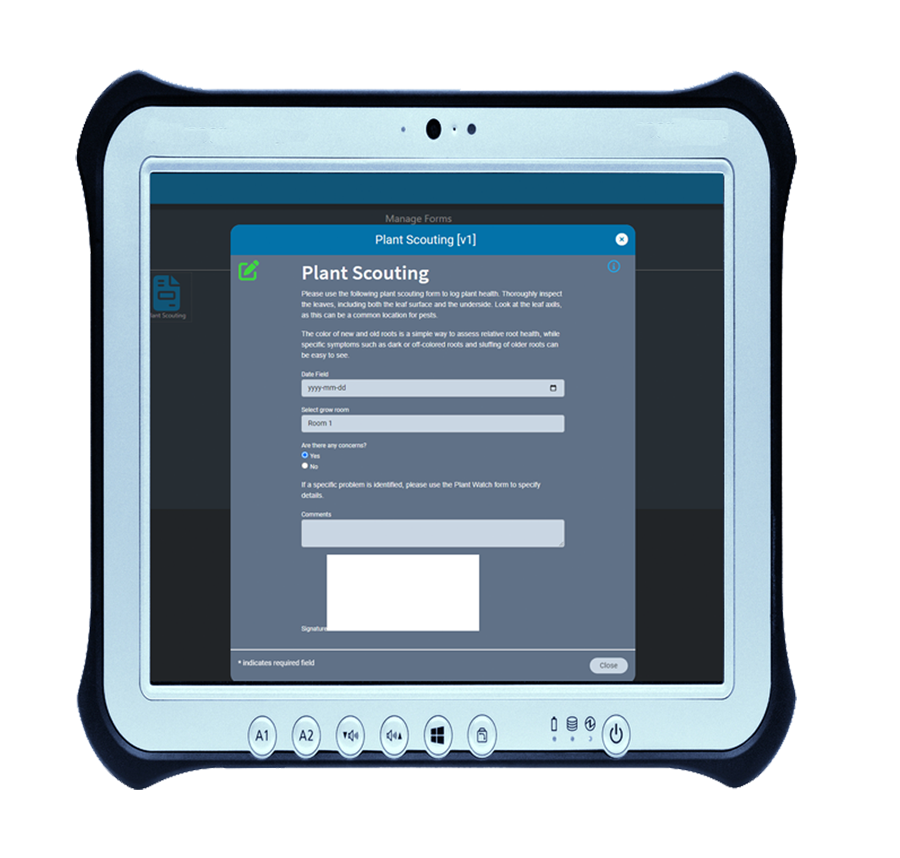
For many compliance-based businesses, forms are unavoidable. Even with the most comprehensive software, forms are sometimes necessary for collection and verification of data.
While many software vendors offer electronic form functionality, in most cases the information contained in those forms is not stored in the database. This means relying on PDFs or printed documents to access the data contained in the form.
Our built-in form designer lets you create custom electronic forms that integrate with AirMed’s system data-sources. All fields in electronic forms created using the form designer can be stored in the AirMed database. There is no need to refer to physical documents or PDFs.
Using AirMed, you can automate workflows using custom e-forms to prevent the need for paper-based documentation. Forms can be linked to processes such as plant scouting, nutrient checks, room sanitation, and shop floor data collection. Using our form designer, you can employ drop-down picklists, date & time, checkboxes, radio groups, custom fields and more. AirMed lets you apply auto-complete, file upload, hidden input and electronic signatures to your forms.
All the data is electronically accessible and even reportable.
Form data collection doesn’t get any easier than that.
For more information visit our AirMed 5 page.
Grow Up Toronto 2024 Conference: May 27-29

Billed as Canada’s #1 cannabis event, the Grow Up Toronto Conference & Expo is a true B2B cannabis event bringing together growers, retailers and suppliers for three days from May 27 to 29.
Grow Up’s Brands and Buyers zone makes its debut in Toronto this year. Brands have the opportunity to promote their latest products to retail buyers and provide samples to qualified cannabis workers.
At the expo, purchasers from the largest retail stores across Canada will converge for two days looking to place orders from the hottest brands from Standard, Micro and Craft processors.
From the latest in technology to workshops with the brightest minds in the industry, combined with the opportunity to sell your brands, Grow Up Conference and Expo is truly an all-in-one stop event for the whole team.
Grow Up is more than just a conference and tradeshow; it’s a pivotal platform dedicated to shaping the future of cannabis in Canada, building strong, long-lasting relationships within the industry.
For more information visit: https://growupconference.com/
Thanks for Making Craft Cannabis Summit a Success!

The BC Cannabis Summit was a success!
Held April 19-21 in Prince George, BC on the traditional territory of Lheidli T’enneh First Nation, this event brought together craft cannabis farmers, processors, independent retailers, Indigenous organizations, government officials, and other sector leaders.
Hundreds participated in this three-day summit that put a spotlight on one of BC’s best-kept cannabis secrets.
In conjunction, the BC Craft Farmers’ Co-op hosted a 4/20 street party on Saturday with 30 vendors and hundreds of members of the public in attendance. This +19 event was the first cannabis-related street closure ever in British Columbia.
AirMed was proud to participate in the summit in Prince George. We’d like to thank everyone who dropped by our booth and especially those who took the time to watch a free demonstration of AirMed software. We appreciate you taking the time to learn about our latest cannabis management innovations.
If you missed seeing a demo or want to see more, click the Request Demo button at the top of the page.
In the meantime, visit our software page: https://airmedcloud.com/airmed-5-intro-new/
For more information about the event visit: https://www.bccraftfarmerscoop.com/2024-bc-craft-farmers-cannabis-summit/
If you missed seeing a demo or want to see more, click the Request Demo button at the top of the page.
In the meantime, visit our software page: https://airmedcloud.com/airmed-5-intro-new/
For more information about the event visit: https://www.bccraftfarmerscoop.com/2024-bc-craft-farmers-cannabis-summit/
To read about the 4/20 cannabis street fair in Prince George, click one of the links below.
High praise for Canada’s first official cannabis street party
Province’s first ever 420 street closure sees huge success in Prince George
Just two days until the Craft Cannabis Summit in Prince George

In just two days, the BC Craft Farmers Co-Op (BCCFC) will hold the 2024 BC Craft Farmers Cannabis Summit in Prince George, BC on the traditional territory of Lheidli T’enneh First Nation.
BC craft cannabis farmers, processors, independent retailers, Indigenous organizations, government officials, and other sector leaders are invited to participate in this three-day summit that will put a spotlight on one of BC’s best-kept cannabis secrets.
While attending the summit, drop by our booth for a free demonstration of AirMed software. We can’t wait to show you our latest software innovations.
For more information visit: https://www.bccraftfarmerscoop.com/2024-bc-craft-farmers-cannabis-summit/
AirMed to Sponsor Growing Relationships Calgary: May 6, 2024

StratCann’s Growing Relationships is back in Calgary this May, and AirMed is a proud sponsor!
Stratcann is passionate about bringing growers, processors, and retailers together to get to know each other and share ideas. Following the success of the sold-out 2023 Growing Relationship series, Stratcann is hosting this one-day event for Alberta’s community of small-batch and micro producers, retailers and other professionals in the cannabis industry.
An important component of the Growing Relationships events is to engage the community in discussion and brainstorming on industry issues from a variety of perspectives.
Attendees will be presented with networking opportunities, engaging panel discussions, a delicious catered lunch, and exclusive access to the Sunalta patio at the Calgary Marriott Downtown Hotel.
For more information visit: https://stratcann.com/growing-relationships/


