ISO, SCC and the Canadian Cannabis Industry

ISO, the International Organization for Standardization, is an independent, non-governmental international organization with a membership of 170 national standards bodies.
Through its members, ISO brings together experts to share knowledge and develop voluntary, consensus-based, market relevant International Standards that support innovation and provide solutions to global challenges.
In Canada, the ISO is represented by the Standards Council of Canada (SCC). A Crown corporation, SCC was established by an Act of Parliament in 1970 to foster and promote voluntary standardization in Canada. SCC is independent of government in its policies and operations, although it is financed partially by Parliamentary appropriation.
When Canada legalized cannabis in 2018, SCC set about creating standards for the new industry believing that standards are essential for an effectively regulated marijuana market. These standards were considered groundbreaking when published in October of 2022.
ISO IWA-37, “Safety, security and sustainability of cannabis facilities and operations” is available for purchase through ISO in three parts.
Part 1 (ISO IWA 37-1:2022) covers requirements for the safety of cannabis buildings, equipment and oil extraction operations. https://www.iso.org/standard/84023.html
Part 2 (ISO IWA 37-2:2022) covers requirements for the secure handling of cannabis and cannabis products. https://www.iso.org/standard/84024.html
Part 3 (ISO IWA 37-3:2022) covers good production practices (GPP). https://www.iso.org/standard/84025.html
Taken as a whole, these documents provide invaluable direction to legislative bodies and emerging companies and help to create a safe, legal market for adults who use cannabis.
For more information from SCC visit: https://www.scc.ca/en/news-events/news/2022/new-guidance-from-iso-international-workshop-safe-cannabis-production
For more information from ISO visit: https://www.iso.org/en/contents/news/2022/10/standards_safe_legal_cannabis.html
To learn more about how AirMed helps you meet these standards, visit our Compliance page.
If you’d like to learn about our quality management and GPP offerings or discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms.
QMS-GPP Planning According to ISO:9000
Introduction
Quality management is the practice of ensuring consistency in products and services throughout your organization. A Quality Management System (QMS) helps your cannabis business provide customers with the best you can offer while mitigating risks using tools that support Health Canada’s Good Production Practices.
A Quality Management System-Good Production Practices Plan provides an overview of the quality management system that an organization has in place. Although there are many standards in the world, ISO:9000 is one of the most respected. And according to ISO:9000 standards, the plan must contain the following.
Quality Policy (ISO:9000 clause 5.2): A quality statement can be derived from a mission statement and/or vision statement, but should explain the organization’s commitment to quality
Quality Objectives (ISO:9000 clause 6.2): These can be the organization’s objectives from a business plan, again, as long as they contain a commitment to quality
Criteria for Evaluation and Selection of Suppliers: As quality management and good production practices are often dependent on supplies and equipment that come from other organizations, organizations need to have a criteria in place for evaluating their suppliers to ensure that they select suppliers that meet QMS and GPP standards
Scope of the QMS: This is a list of all SOPs with brief descriptions/purposes
Quality Product Statement
ISO:9000 requires your organization’s quality policy to be appropriate to your organization’s strategic direction and operational direction (context).
Your organization must understand and identify all the influences that affect its business and ensure that the strategy and direction takes quality into consideration. Your organization will need to review its current quality policy regularly to ensure that any changes in context, interested parties or other requirements are reflected, and to determine whether your organization’s objectives are affected. (ISO 9001:2015 – 6.2.1a.)
Following is an example.
Company ABC produces cannabis products for distribution in Canada according to the regulations in the Cannabis Act. The Company has developed its production system through experience and its aim is to achieve a high standard of production and products to its customers.
It is the policy of Company ABC to provide the customer with goods to the agreed requirement in accordance with the details and price.
The Directors, Management and Staff are responsible for Quality Control through the Quality Management System seeking improvement by constant review, with suppliers and sub-contractors being encouraged to co-operate. The Company is committed to achieving customer satisfaction by the use of quality procedures which will be operated to meet or exceed the requirements of [the Cannabis Act and/or ISO 9001 or other quality system].
Quality Objectives
The quality objectives should act as a driver for continual improvement. To meet quality standards, your organization will be required to ensure that you continually improve products and services to meet customer requirements and to measure effectiveness of the processes responsible.
Following is an example.
Company ABC strives to be the best provider of cannabis products in Canada. Through the use of this guiding principle, everyone in Company ABC is accountable for fully satisfying our customers and authorities by meeting or exceeding their needs and expectations with best-in-class production practices. Our goal is 100% customer satisfaction and compliance 100% of the time.
Our Quality Policy is defined and strongly driven by the following objectives:
1. Meet all compliance requirements for all levels of governments and regulatory agencies
2. Build a mutually profitable relationship with our customers, ensuring their long-term success, through the understanding of their needs and the needs of their customers as well
3. Achieve our commitments for quality, cost, and schedule
4. Use of best preventive practices at all levels and ensure reliable risk management
5. Drive continual improvement and innovation based upon efficient business processes, well-defined measurements, best practices, and customer surveys
6. Develop staff competencies, creativity, empowerment and accountability through appropriate development programs and show strong management involvement and commitment
Evaluation and Selection of Suppliers
Supplier evaluation is a system for recording and ranking the performance of a supplier in terms of a variety of criteria and is a must in ISO:9000. A process of vendor rating is essential to effective purchasing. While there is no one right system for supplier evaluation and selection process, the overall objective is to reduce risk and maximize overall value to the purchaser.
Criteria
There are eight common supplier selection criteria:
1. Cost
2. Quality & Safety
3. Delivery
4. Service
5. Social Responsibility
6. Convenience/Simplicity
7. Risk
8. Agility
In the cannabis industry, you should also add a commitment to meeting compliance and/or helping their customers meet compliance.
Methods
There are many other methods of evaluation, and the organization should determine which is the best for its use.
Categorical systems typically use excellent, good, average, poor and so on.
Weighted systems rate on a scale from 1 to 10 or out of 100.
Hierarchical systems give values in relation to each item’s importance. The most important item is given the highest value.
Conclusion
Of course, a quality management system and good production practices plan is only as good as the processes that support it. Creating standard operating procedures and ensuring that all personnel follow them will give you the best chance of success.
For more information about ISO:9000 visit: ISO – ISO 9000 family — Quality management
For information on how AirMed helps you meet compliance, visit our Compliance page.
If you’d like to learn about our quality management and GPP offerings or discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms.
SOP Library & Editor in AirMed5
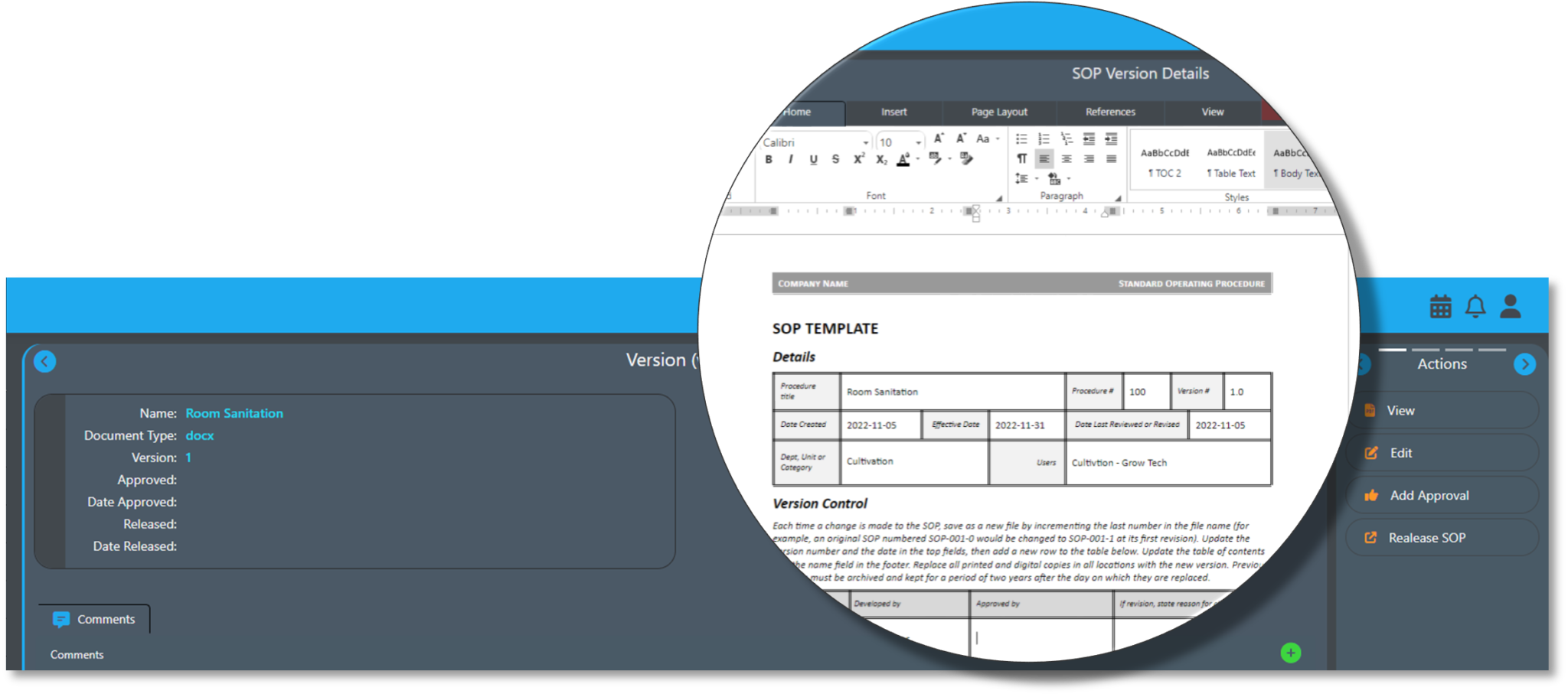
AirMed5 offers management for standard operating procedures with a built-in SOP editor as part of the new Quality Management System (QMS).
A new SOP library with automated version management lets you track current, historical and draft versions of standard operating procedures used in your facility.
A built-in SOP editor lets you create or edit document content and formatting without leaving AirMed. Users can modify SOPs, send for review and employ an approval process to verify changes before replacing an existing SOP with a new version.
For more information on these new features or to book a demo of AirMed to see them for yourself, click the Request Demo button at the top of the page or use any of the contact forms.
In the meantime visit https://airmedcloud.com/airmed-5-intro/
Quality Management in AirMed5
Multi-level Approvals
AirMed5 includes the option to record eSignatures and lets you create an approval workflow with one or more signatures required for a given activity.
Approval workflows can include a single signature or one or more unique signatures plus optional witnesses. When an individual is tasked with electronically signing for an approval, a notification is sent through the AirMed internal messaging system. Within the message is a quick link to the location where the eSignature is required.
QMS Incident Tracking
A new incident management system lets users record incidents that deviate from normal operating protocols or that affect compliance data. Incident tracking can be used in any area of the facility including plant growth, extraction, orders and fulfillment.
Incidents can be automatically created when certain events occur, such as back-dating or undoing/editing specific data. AirMed creates a comprehensive audit trail for any alteration made to compliance data and provides auditors with a tool to examine corrections or modifications.
Escalation & Investigations
Escalation of specific types of incidents can be automated using message notifications. Users can initiate an inquiry related to an incident and have it automatically escalated to an appropriate investigator.
Investigations could be related to any incident such as employee illness or injury, hazardous spills, deviations, non-conformance, loss or theft, recalls, vender audits, crop destruction, pests, or any other incidents. AirMed manages each incident through the cycle of investigation, evaluation, resolution, corrective action, and procedures.
For more information on these new features or to book a demo of AirMed to see them for yourself, click the Request Demo button at the top of the page or use any of the contact forms.
In the meantime visit https://airmedcloud.com/airmed-5-intro/