Stratcann’s Growing Relationships Event Series

Growing Relationships is StratCann’s signature event series that brings together producers, retailers, and service providers in the cannabis industry for conversation, innovation, and collaboration. This B2B event is a full day experience, with a focus on the region’s independent cannabis brands and businesses.
Upcoming events in 2025 include:
- July 28: Mission BC
- August 15: Moncton NB
- August 19: Ottawa ON
- September 19: Nanaimo BC
- October 6: Kelowna BC
For more information visit: https://stratcann.com/growing-relationships/
AirMed: Scalable Software for Your Cannabis Business

Last week we discussed building a scalable and sustainable business. Read the post here: Sustainably Scaling Your Cannabis Business.
Creating a cannabis business that is both scalable and sustainable is the best way to deal with an uncertain economy. One of the keys to scalability lies in having tools that can handle a varying volume of transactions, inventory, and data.
Our cannabis management software is designed with scalability in mind. Whether you’re a startup or a large enterprise, our platform grows with you.
Scaling up operations can mean more complex processes, such as adding new product lines, expanding to new locations, or hiring additional staff. AirMed lets you handle these complexities with ease. Features such as multi-location support, role-based access, and centralized reporting help you streamline processes and avoid bottlenecks.
AirMed manages every aspect of cultivation, processing, packaging, and distribution. We also offer quality management, workforce management, CRM and ERP-level functionality.
You can automate a series of tasks and assign them to different departments or users with activities cascading across the entire production cycle. Plan templates let you create formulas after completing the cultivation, drying and packaging of a successful batch. Our software automates inventory management and monitors stock levels in real-time to let you adjust to fluctuating demand.
With integrated tools for sales reporting, AirMed can provide data-driven insights to help you make smarter decisions. Whether it’s forecasting sales trends, identifying popular products, or tracking seasonal demand, data becomes even more valuable as the economy fluctuates.
Our system is module to let you add functionality if and when needed. We also offer flexible billing options, and we’re currently developing a lower-cost ‘lite’ version of AirMed to help micros. A limited feature set and simplified workflows will get your smaller operation up and running while ensuring that you meet compliance and stay within budget.
Investing in scalable software ensures that your business can handle changing demand, variable inventories, and flexible operations to set the stage for long-term success. With the right system in place, you can focus on your business, while knowing that your operations are efficient, compliant, and sustainable.
For more information visit our Software page or our Compliance page.
Sustainably Scaling Your Cannabis Business
As the cannabis industry fluctuates, businesses must be prepared to scale operations depending on demand. Building a scalable, but also sustainable, cannabis operation is about more than just sales or product. It’s about creating a foundation that allows your business to deal with varying volumes of transactions, inventory, and data without introducing inefficiencies or compliance risks. Your operations should be flexible enough to accommodate your current demands while being adaptable to whatever the future has in store.
Scaling Up
During upturns, your sales volume and inventory size will inevitably increase, so your operations must be able to handle that. Your production may become more complex as a result, and you may want to expand. Whether you’re adding new product lines, opening new facilities, or hiring additional staff, be conscious of overbuilding infrastructure in case of a downturn. If you do decide to invest, look for systems that will support long-term efficiency. And ensure that whatever you scale up can be scaled back if needed.
1. Increase operations incrementally or modularly. Add capacity in blocks based on actual demand, or partner with those who can provide what you lack to reduce up-front costs.
2. Maintain your cash flow and build reserves during growth phases to cushion slumps. Avoid overleveraging and watch your ‘burn rate.’ Scale in ways that don’t push your operating expenses to unsustainable levels.
3. Grow where you’re strongest. Deepen presence in high-margin, high-loyalty markets before chasing expansion. Defend your base by building brand equity and customer loyalty with retention strategies before aggressively acquiring new customers or markets.
4. Diversify selectively. If you want to diversify, offer a mix of premium and budget-friendly options to serve customers in both boom and bust conditions. If feasible, expand into sectors or geographies less exposed to fluctuations.
5. Invest in people, culture, and leadership. For long-term sustainability, hire selectively and cross-train to build a team that can wear multiple hats if needed. Encourage adaptability, learning, and resourcefulness. Create strong leaders, a key to weathering uncertainty.
Scaling Down
During a slow economy, the best way to deal with a situation that seems beyond your control is to ensure that you have control over everything you can.
1. Streamline operations and optimize cultivation to reduce waste and increase profits. For example, using data analysis on lighting, nutrients, and water usage can increase yield without compromising quality.
2. Prioritize high-margin products and markets. Evaluate strains, product formats (e.g., pre-rolls, vapes), and brands to determine which sell reliably and offer the healthiest margins. Increase or reduce efforts in specific markets depending on performance metrics.
3. Incentivize loyalty. Offer discounts, product education, or other incentives to existing clients and partners. Encourage pre-paid orders or early payment discounts to improve cash flow.
4. Communicate transparently. Be upfront with all stakeholders about the current conditions. Encourage ideas from frontline workers on efficiency and even company direction, and show investors you’re taking disciplined, strategic action.
5. Utilize scalable systems and tools. Your equipment, software and services should scale with your business needs. Discuss temporary options with your vendors such as reduction of services to control short-term costs while maintaining long-term relationships.
Conclusion
By staying agile and innovative, you can pivot quickly when needed. And by utilizing all available data, you can improve decision making throughout your operations. This will help you invest selectively in growth-enablers to give you the best return on investment (ROI) whatever the economy throws at you.
For more information visit our Software page or our Compliance page.
Profile: ABLE BC, the voice of BC’s cannabis stores

The Alliance of Beverage Licensees (ABLE BC) is the voice of British Columbia’s bars, pubs, and private liquor and cannabis stores.
A non-profit organization funded by membership dues, ABLE’s mission is to help British Columbia’s liquor and cannabis businesses succeed.
On behalf of the organization’s 1,000 members, ABLE BC works with all levels of government to create business-friendly policies and enhance private sector opportunities.
Headquartered in Vancouver, the association offers advocacy work, member benefits and discounts, and direct access to the latest industry information and policy expertise for cannabis retail members.
According to the organization’s website, ABLE BC “works hard every single day to advocate for your interests, protect your businesses, and help ensure the survival of the cannabis retail and private liquor industries.”
For more information and to sign up for ABLE BC’s free newsletter, visit:
https://ablebc.ca/
Cannabis Europa 2025: June 24-25 in London, England

Join 1,500+ influential leaders from thriving cannabis companies, investors actively deploying capital into the market, plus key politicians — the crucial combination needed to drive the European cannabis industry and your business forward.
Cannabis Europa will once again be holding a boutique industry expo, showcasing more than 40 leading businesses as the cream of the European cannabis crop, acting as a jumping off point for growing your business in Europe and beyond.
Cannabis Europa is where the visionaries, policymakers, and business leaders shaping Europe’s cannabis landscape will converge. 2025’s conference will explore Europe’s distinct path forward, emphasising clinical excellence, regulatory integrity, and a focus on sustainability that sets the region apart as a global benchmark for cannabis standards.
For more information visit: https://www.cannabis-europa.com/
AirMed & Cannabis Inventory Management

Last week we discussed the benefits of inventory management. Read the post here.
This week, we’ll look at ways that AirMed helps streamline inventory management for cannabis businesses.
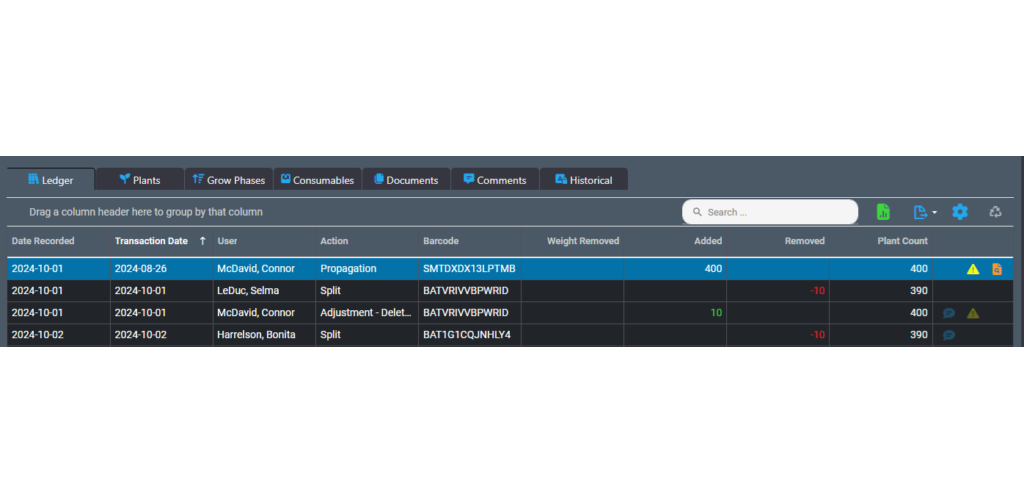
In 2024 we introduced ledgers designed to streamline your inventory management and recordkeeping.
AirMed integrates inventory ledgers throughout the system letting you effortlessly track all transactions related to your inventory. Our inventory ledgers provide detailed tracing of every addition or reduction in batches or lots with full audit trails. We also added intelligent backdating to ensure adjustments or entries made in the past won’t create discrepancies in the future. And we’ve made it easy for you to resolve inventory inconsistencies by offering multiple tools for making corrections.
But ledgers are not the only inventory management tools in AirMed.
Our software offers GS-1 barcoding with full functionality for multi-level packaging that lets you assemble retail-ready individual packages using a ‘master case’ system and identify & track products at each packaging level.
AirMed offers unparalleled ease while dealing with complex order requirements. Manage order requests, order planning & assembly, as well as shipping from one screen. After releasing a planned order to production, verify order items in one step. You can select available inventory from multiple lots for order fulfillment. Browse products, package sizes and available inventory, then initiate package runs and pack cases workflows in the items table.
Since AirMed reports on every item in the system, inventory reporting is available at the click of a button. Real-time inventory tracking lets businesses assess product demand more effectively, reducing excess inventory and lowering costs associated with unsold goods.
Utilizing cannabis inventory management is more than just a way to keep track of products—it’s a key to reducing losses, improving operational efficiency, and boosting profitability.
AirMed has been 100-percent Canadian owned and operated since it was created in 2014. Click the Request Demo button at the top of the page today to explore AirMed in a free walkthrough and learn what home-grown can do for you.
For more information about AirMed visit our Software page.
To read more about our inventory ledgers, read our previous posts.
AirMed 5 Introduces Inventory Ledgers
Streamlining Cannabis Inventory Management

Effective inventory management is crucial in the cannabis industry. With strict regulations governing everything from product tracking to distribution, any inefficiencies or inaccuracies can result in legal issues, loss of product, or lost revenue.
Cannabis businesses, whether cultivators, manufacturers, or retailers, are tasked with managing complex inventory systems that require real-time updates, accurate tracking, and compliance with provincial and federal laws. This is where cannabis management software can come into play.
Software that provides advanced inventory management functionality helps automate and streamline processes, while tracking every product, at every stage.
With features like barcode scanning and real-time inventory updates, cannabis businesses can gain a clear, up-to-date view of their inventory. This transparency helps to minimize human error and avoid overstocking or stockouts—both of which can have serious financial implications.
Inventory management can streamline processes like harvesting, trimming, packaging, and distribution, reducing resources. A good inventory management system can also detect shrinkage from spoilage, mislabelling or theft for better cost control and waste reduction.
Inventory data can be used to anticipate demand, plan crops, or shift production toward high-margin products. A producer can improve supply chain decisions and lead times for restocking. Meeting demands without delays and ensuring consistent availability across dispensaries or partners ultimately results in enhanced customer satisfaction.
While operational efficiency is the most obvious result of streamlining inventory management, by providing insights into trends and sales patterns, businesses can make data-driven decisions to optimize all processes and increase profits.
AirMed has been 100-percent Canadian owned and operated since it was created in 2014. Click the Request Demo button at the top of the page today to explore AirMed in a free walkthrough and learn what home-grown can do for you.
For more information about AirMed visit our Software page.
Health Canada Seeks Feedback on Natural Products with Cannabidiol by June 5

A consultation is under way by Health Canada called: Towards a pathway for health products containing cannabidiol. The goal of this public consultation is to consider permitting cannabidiol (CBD) as a medicinal ingredient in natural health products. This would require amendments to Schedule 2 of the NHPR and the PDL.
“If these amendments are made, Health Canada would have the ability to regulate NHPCCs under the NHPR [natural health products containing cannabidiol]. Provided that a product licence application for an NHPCC meets the licensing requirements in the regulations, such products could then receive a natural product number (NPN). This number indicates that Health Canada has reviewed and authorized a product.”
In the consultation document, Health Canada states, “There has been continued interest from stakeholders to include CBD in health products available without a prescription and consumers who want to access them. As a result, Health Canada committed to looking at a potential regulatory pathway for NHPCCs that may be accessed without a prescription.”
The consultation began in March and the deadline for submission of feedback is June 5, 2025.
For more information on this consultation including how to participate visit:
https://www.canada.ca/en/health-canada/programs/consultation-towards-pathway-products-containing-cannabidiol/natural-health-products.htmlFor an in-depth analysis of this consultation visit Stratcann:
https://stratcann.com/news/health-canada-seeks-more-feedback-on-cbd-as-a-natural-health-product-and-cbd-for-pets/
Grow Up Toronto 2025 Conference: May 27-28

Grow Up is returning to Toronto from May 27 to 28, 2025, and bringing back the highly anticipated CannaVision Executive Summit.
Join industry leaders, growers, and cannabis professionals for this dynamic event, where you’ll explore cutting-edge trends, global cannabis markets, and the future of the industry.
With insightful panels, networking opportunities, and the best in cannabis innovation, Grow Up Toronto 2025 promises to be an event you won’t want to miss.
Grow Up Conference is a privately owned Canadian company since 2017 focusing on cultivation, brands and retail. You will be surrounded by industry professionals with a lot of opportunities to make new connections.
For more information visit: https://growupconference.com/
AirMed & Product Diversification
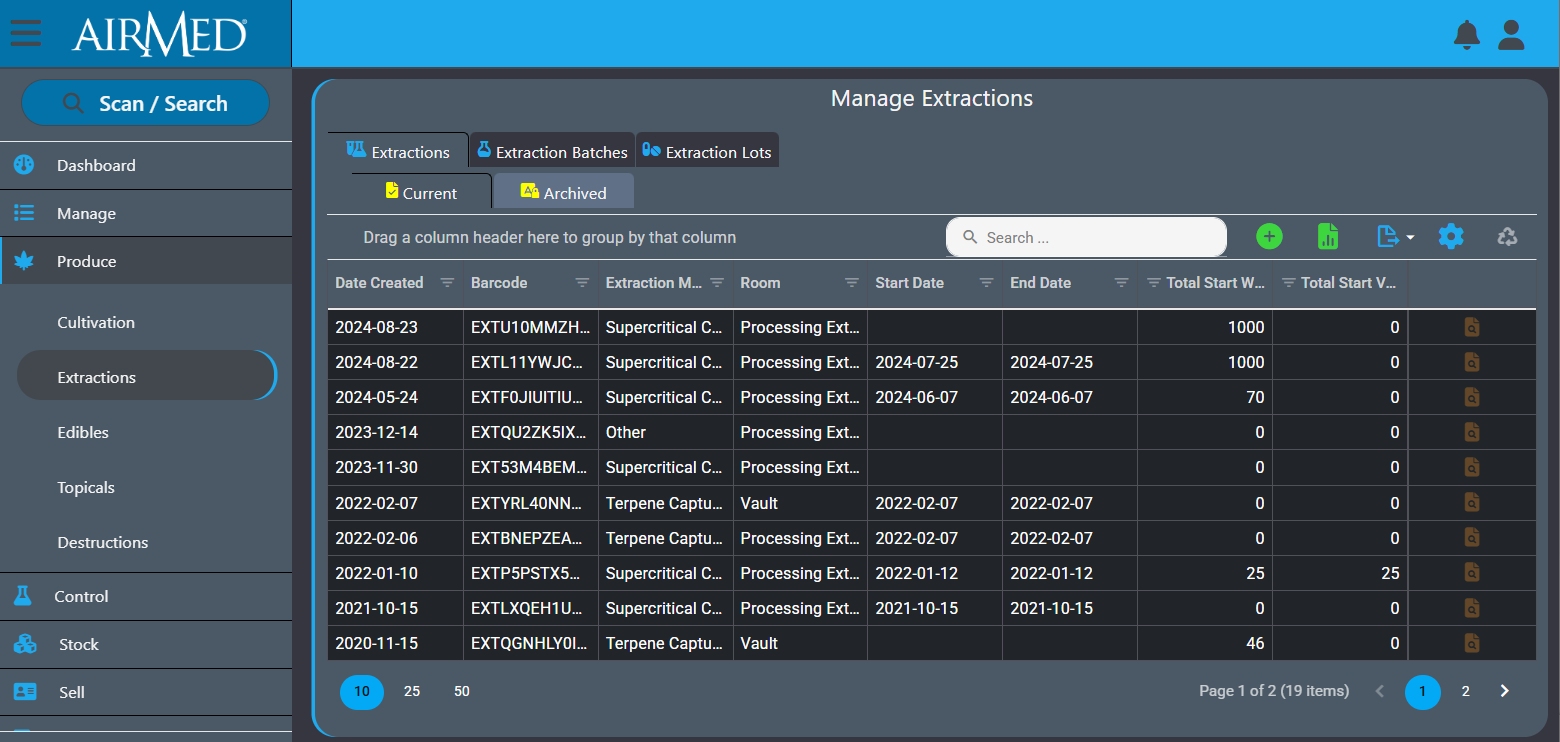
In a recent post, we discussed the benefits of product diversification for cannabis producers. Read the post here: https://airmedcloud.com/benefits-product-diversification/
If you have a diversified product range or are considering diversifying, know that AirMed is here for you. No other cannabis management system offers the breadth of functionality, ease of use, and comprehensive features to cover all classes of cannabis.
- Dried cannabis
- Fresh cannabis
- Cannabis plants
- Cannabis plant seeds
- Cannabis edibles
- Cannabis extracts
- Cannabis topicals
Some cannabis products, such as extracts, edibles and topicals, are categorized as special classes due to the unique public health and safety risks they present. As a result, these have specific requirements pertaining to their formulation, production and composition. AirMed offers features designed specifically for those cannabis classes. There are dedicated workflows for storing extractions after processing for further processing or for blending with other materials and more. Weights are tracked to five decimal places for precision and reporting.
Packaging features in AirMed include the ability to create discrete units such as edible gummies or gel caps, which can be recorded individually in groups or as bulk packages.
Our system offers GS1 barcoding and configuration for tracking product cases and master cases. And all labels are produced and created from within AirMed and can be customized to your needs.
With more stringent requirements for some cannabis classes comes a greater need for control and risk mitigation. Access to AirMed software functionality is controlled on a user-by-user basis. Workforce management in our software utilizes configurable departments and job roles to control which workflows workers are authorized to use. You determine who is given rights to these production areas based on the needs of assigned job roles.
We’ve also provided a range of quality management (QMS) features to support Good Production Practices (GPP) and a Preventive Control Program (PCP).
To maintain compliance with Health Canada and accurately complete the monthly CTLS report, licenses processes have specific data management requirements. We’ve already accommodated those needs with data automatically populated in the CTLS Worksheet Tool.
AirMed not only accommodates compliance reporting but business intelligence as well. Hundreds of pre-built reports are available for sales, clients, inventory, production and more.
And AirMed can manage multiple client facilities, and data can be partitioned to each respective facility.
AirMed has been 100 percent Canadian owned and operated since it was created in 2014. Click the Request Demo button at the top of the page today to explore AirMed in a free walkthrough and learn what home-grown can do for you.
For more information about AirMed visit our Software page.
The Benefits of Product Diversification

A Strategic Advantage for Producers
Cannabis producers are continually seeking ways to stay competitive and expand market share. One strategy for achieving these objectives is through diversification, which can include edibles, extracts, topicals, and even non-psychoactive CBD products. Diversification not only mitigates many of the risks associated with market volatility but also presents opportunities for innovation, efficiency and sustainability.
Risk Mitigation: The market for cannabis is subject to external pressures such as regulatory changes, shifts in consumer preferences, and economic fluctuations. By developing a range of products, manufacturers can buffer against these uncertainties. If one product line faces a downturn due to new regulations or market saturation, having a portfolio of alternative products can help maintain revenue streams.
Market Expansion: Diversification lets cannabis producers tap into different market segments to increase their chances of capturing market share in an expanding and competitive landscape. The cannabis market is no longer limited to flower smokers; consumers now seek items tailored to their preferences, such as edibles, tinctures, topicals, concentrates, and even beverages or health-focused CBD products. Each product range appeals to different demographics, from those who prefer discreet consumption methods to those interested in recreational or medicinal benefits. By offering a broad spectrum of products, producers can attract a wider consumer base, including those who might not typically engage with traditional cannabis products.
Innovation & Brand Loyalty: By innovating, producers can set trends rather than follow them, creating unique offerings that can become synonymous with their brand. This not only helps capture market attention but can also build strong brand loyalty. Consumers are more likely to remain loyal to a brand that consistently offers new, exciting, and effective products. Customers who have a good experience with a flower product may gain the confidence to explore edibles, tinctures, or wellness items from the same producer. Offering a wide variety of high-quality products enables cannabis producers to build stronger relationships with consumers. Moreover, diversifying helps a brand stand out from competitors, positioning it as a one-stop shop. Building an extended portfolio reinforces brand reputation and credibility in a market where consumer trust is essential.
Efficiency & Sustainability: Diversifying product lines can lead to better utilization of resources and contribute to environmental sustainability. Different products might use different parts of the operation, ensuring that waste is minimized, and all plant materials are used more efficiently. This can lead to economies of scale, potentially lowering the cost per unit and reducing the environmental footprint, which can appeal to environmentally conscious consumers.
Compliance with Evolving Regulations: The cannabis regulatory landscape is continuously changing, with regional laws specifying what types of products can be sold. By diversifying, producers are better positioned to adapt to regulatory changes. If one jurisdiction bans smoking, a producer with a line of edibles or topicals can still serve that market, maintaining presence and revenue.
Conclusion
Diversification offers a way to manage risk, expand market reach, foster innovation, optimize resources, comply with regulations, and contribute to sustainability. For cannabis producers looking to thrive, diversifying can be the answer. By expanding portfolios, producers can reach new customers, reduce financial risks, stay ahead of trends, and increase profitability. As the industry continues to mature, those who embrace product diversification will likely lead the market, setting standards and capturing the diverse needs of consumers worldwide.
AirMed has been 100 percent Canadian owned and operated since it was created in 2014. Click the Request Demo button at the top of the page today to explore AirMed in a free walkthrough and learn what home-grown can do for you.
Watch for an upcoming post on how AirMed handles a range of cannabis products and classes. In the meantime, visit our Software page.
Report: Cannabis Cultivation Market 2025

The Cannabis Cultivation market report covers market characteristics, size & growth, segmentation, regional & country breakdowns, competitive landscape, market shares, trends and strategies for this market.
This is one of a series of reports on the cannabis industry published by Research and Markets, an industry analysis firm headquartered in Dublin Ireland.
The analysts at Research and Markets predict the cannabis cultivation market will grow from $179.32 billion in 2024 to $208.64 billion in 2025 at a compound annual growth rate (CAGR) of 16.3%. “The growth in the historic period can be attributed to legalization trends, medical use acceptance, consumer awareness, investment inflow.”
The reports goes on to predict the cannabis cultivation market “will grow to $407.91 billion in 2029 at a compound annual growth rate (CAGR) of 18.2%. The growth in the forecast period can be attributed to research and development, sustainable practices, consumer education.”
Although the full 200-page report is expensive, the description and executive summary are free and provide excellent information on the marketplace including the following points.
- The adoption of cannabis for treating chronic diseases is anticipated to drive the growth of the cannabis cultivation market in the future.
- The increasing public acceptance and demand for cannabis are expected to boost the growth of the cannabis cultivation market going forward.
- Product innovations are a key trend gaining popularity in the cannabis cultivation market.
To read the description & executive summary of this report, visit:
https://www.researchandmarkets.com/reports/5766626/cannabis-cultivation-market-report
Canadian Cannabis Research Summit: Apr 30-May 1, 2025 in TO

The 2025 Canadian Cannabis Research Summit will bring together the largest assembly of cannabinoid researchers and clinicians in Canada in over a decade. This landmark event will celebrate Canada’s rich diversity of cannabinoid research, shining a spotlight on the research centres, networks, and groups driving innovation in this critical field.
This national gathering of Canadian cannabinoid medicine researchers, clinicians, trainees and allied healthcare practitioners will showcase research being conducted from coast to coast. This event will also continue detailed discussions on the latest cannabinoid research, clinical care and future government policies.
The summit is hosted by the Canadian Consortium for the Investigation of Cannabinoids (CCIC), whose mission is to advance and promote evidence-based research and education concerning the endocannabinoid system, the therapeutic applications of cannabinoids, the potential harms associated with cannabis use and the societal and health impacts of non-medical cannabis use to the health care community and the general public. The CCIC represents a group of researchers, healthcare professionals and educators who promote a balanced perspective regarding the current science says about the biological, health and societal effects of cannabis and cannabinoids.
This event promises to be Canada’s most comprehensive forum on the science behind cannabis, its potential therapeutic benefits and the societal and health impacts of legalization of non-medical cannabis from leading researchers and physicians in the field.
April 30 and May 1, 2025 at Victoria College, 73 Queen’s Park Crescent in Toronto, ON. For more information visit: https://ccic.net/ccic-conference/
Health Canada Revises Certain Cannabis Regulations

For the purposes of streamlining requirements, Health Canada has amended certain regulations concerning cannabis.
Canadian cannabis news site Stratcann published an article on March 12 discussing the revisions.
“The regulation changes focus on five key areas: licensing, production, packaging and labelling, security, and record keeping. The government’s goal with these changes is to address some of the challenges expressed by the industry while maintaining the key public health and safety concerns within the federal Cannabis Act.”
In the new regulations, which were made official in February and took affect last week, production limits for micros increased, and requirements for non-human and non-animal research testing decreased.
Another revision omitted the printed “Consumer Information Document” requirement that in the past had to accompany every shipment of cannabis in Canada.
There were also revisions for hemp producers.
Read a summary revised regulations here: https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/summary-changes-following-streamlining-regulations.html
Read the Stratcann article on the changes here: https://stratcann.com/other/updates-cannabis-rules-regulations-now-public/
Still Proudly Canadian-Owned and Operated

After more than 10 years in business, we are incredibly proud to remain 100 percent Canadian-owned and operated. From day one, our mission has been clear: to provide world-class products and services while staying true to our Canadian roots.
Every decision we make is guided by our commitment to support the Canadian cannabis industry — from Cape Scott to Cape Spear and every point in between.
With hard work, innovation, and the continued loyalty of our customers, we’ve built something truly special and truly Canadian.
Through our second decade in business, we hope to continue serving you with the same passion, dedication, and Canadian pride that has defined us since 2014.
Take a tour of AirMed to see what homegrown can do for you. Call us today at (877) 313-2442 or use the Request Demo button at the top of the page.
In the meantime, visit our Software page.
Study: Medical Cannabis in Older Patients

A study carried out by representatives of the University of Victoria and the commercial cannabis industry reports that “Approximately 90% of patients used medical cannabis to treat pain-related conditions such as chronic pain and arthritis. Almost all patients reported a preference for oral cannabis products (e.g., extracts, edibles) rather than inhalation products (e.g., flower, vapes), and most preferred oral formulations high in cannabidiol and low in tetrahydrocannabinol.”
This study aimed to assess the patterns of medical cannabis use in patients over 50 years of age and its effect on health outcomes such as pain, sleep, quality of life, and co-medication.
The Medical Cannabis in Older Patients Study (MCOPS) had treating physicians collecting detailed data on participant characteristics, medical cannabis and co-medication use, and associated impacts on pain, sleep, quality of life, as well as adverse events.
There were 299 participants with an average age of 66.7 years, 66.2% of which identified as female.
“Over the six-month study period, significant improvements were noted in pain, sleep, and quality of life measures, with 45% experiencing a clinically meaningful improvement in pain interference and in sleep quality scores. Additionally, nearly 50% of patients taking co-medications at baseline had reduced their use by the end of the study period, and quality of life improved significantly…”
The study was published by the Research Society on Marijuana and can be accessed here:
https://publications.sciences.ucf.edu/cannabis/index.php/Cannabis/article/view/239An article summarizing the results was published on Norml.org, an organization whose mission is to move public opinion sufficiently to legalize the responsible use of marijuana by adults, and to serve as an advocate for consumers to assure they have access to high quality marijuana that is safe, convenient and affordable.
https://norml.org/blog/2025/02/21/study-cannabis-use-in-older-patients-associated-with-improved-quality-of-life-lower-demand-for-prescription-drugs/
CannExpo 2025: Mar 21-22 in Toronto

CannExpo is the ultimate event for professionals, consumers, budtenders, LPs, start-ups, retailers, investors, and curious newcomers in the cannabis retail sector.
Dive into the heart of the cannabis industry, and explore new brands, connect with the cannabis community, and learn from industry leaders at CannExpo.
This event will be held March 21-22, 2025 at the Queen Elizabeth Building, Exhibition Place, Toronto. For more information visit: https://cannexpo.ca/
Data-Driven Decision Making & AirMed
Data-driven decision-making is the practice of using facts, metrics, and data to guide strategic business decisions.
From optimizing cultivation processes to enhancing marketing strategies, the ability to analyze and act on data can make the difference between thriving and struggling in this dynamic cannabis space. By leveraging data, cannabis operators can gain actionable insights, reduce costs, improve yields, ensure compliance, and ultimately achieve a competitive edge.
Benefits of Data-driven Decision Making
Cannabis cultivation is a complex process influenced by numerous variables. By using data analytics, producers can monitor these variables in real-time, identify trends, and make adjustments to maximize yield and quality.
Cannabis is a resource-intensive product. Data-driven strategies help identify inefficiencies and optimize resource allocation. This ensures that resources are used as effectively as possible and reduces waste. These measures not only decrease operational expenses but also support sustainability initiatives—an important consideration for environmentally conscious consumers.
Compliance requires meticulous record-keeping and traceability throughout the supply chain. Data-driven systems that leverage information to demonstrate adherence to quality and safety standards enhance credibility with regulators and customers alike. Moreover, predictive analytics can help identify potential risks, enabling proactive measures to mitigate them.
As the cannabis industry matures, innovation is key to staying ahead of competitors. Data-driven insights can empower you to experiment with new cultivation techniques, product formulations, and market strategies. By staying informed and agile, you can seize opportunities and respond effectively to market shifts.
With narrow profit margins and significant operational costs, data-driven decision-making can also support financial sustainability. Data analytics can provide insights into cost drivers, helping you identify areas where they can reduce expenses or increase efficiency. Analyzing labor costs and production timelines might highlight opportunities to streamline operations, while inventory data can ensure that stock levels align with demand, reducing the risk of overproduction or stockouts.
AirMed & Your Data
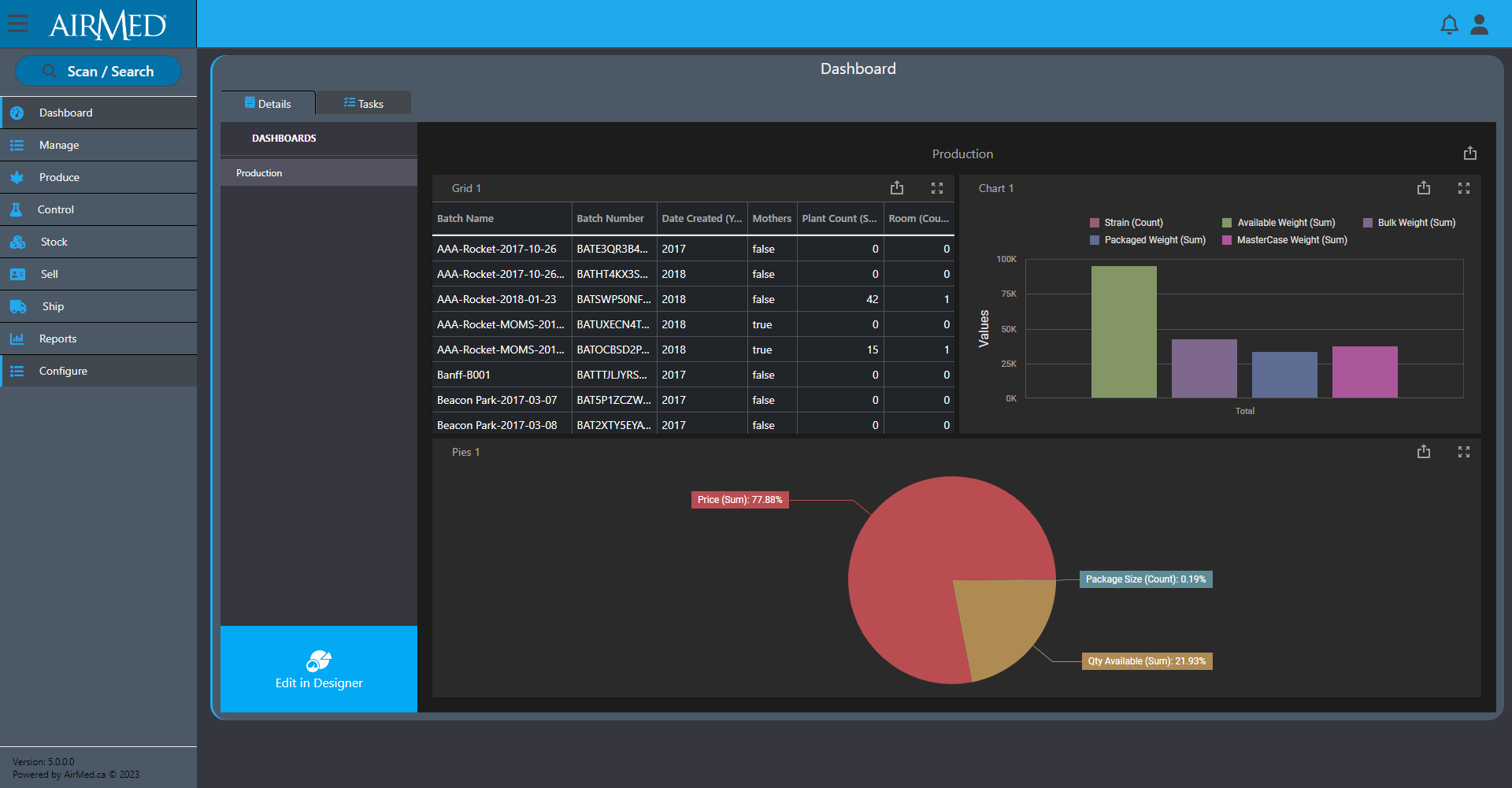
AirMed has always gone beyond other systems by tracking and reporting thousands of fields of data.
Dozens of reports come standard with AirMed, but we also offer an optional report designer to create unlimited custom reports with access to every field of data in the system. You can select individual fields to include in a report and add options to manage master-detail relationships, cross-tab reports, table and vertical reports, and filter options.
And our optional business intelligence (BI) designer provides pre-designed dashboard widgets that offer the best data visualization option for you. You can create insightful and information-rich decision support systems by simply selecting the appropriate UI widget: Chart, Pivot Table, Data Card, Gauge, TreeMap, Map, Grid, or simple Filter elements. By dropping data fields, results are immediate, accurate and always relevant.
For those who wish to edit or create new widgets, the BI Dashboard is engineered to let you spend more time on business and less on UI customization. Whether it’s manipulation of individual chart series, specifying a pivot table’s dimensions or connecting UI elements to fields across different data-sources or data providers, the BI Dashboard designer is built to make your experience a productive one.
Conclusion
Incorporating data-driven decision-making provides the ability to collect, analyze, and act on data to optimize processes, improve product quality, reduce costs, ensure compliance, and remain competitive. AirMed has been designed to help you embrace a data-driven approach.
For more information visit our Software page.
Report: Global Cannabis 2024-2028

The Global Cannabis Report: 5th Edition delivers a thorough analysis and insight into the world’s key cannabis markets, organised by region. This edition explores the global evolution of cannabis markets and examines possible future developments on the international stage. Additionally, it highlights the most notable and relevant trends in the industry, offering regional insights, market sizing data and analyses, and exclusive interviews with cannabis industry experts from around the world.
This report is produced by Prohibition Partners, which provides specialist information, data analytics and digital commerce solutions to the B2B cannabis industry.
For more information and to download the ‘Lite” version of the report for free, visit:
https://prohibitionpartners.com/reports/the-global-cannabis-report-5th-edition/
SPARK BC Conference: March 5, 2025 in Vancouver

SPARK 2025 is hosted by the Alliance of Beverage Licensees (ABLE BC), the voice of British Columbia’s bars, pubs, and private liquor and cannabis stores.
An electric convergence of industry leaders in cannabis and liquor retail make this event your chance to fuel innovation, share expertise, and drive growth in your operations.
Covering a wide range of topics including advocacy, community safety, staff retention, service excellence, growth, regulations, and more, this is your comprehensive resource for innovation and connection.
With carefully designed sessions and engaging speakers, SPARK 2025 will give you the tools to accelerate your business operations in the private liquor & cannabis industries.
Join ABLE BC on Wednesday, March 5, 2025, at the Sheraton Vancouver Wall Centre for BC’s premier liquor & cannabis conference.
For more information on the conference visit: https://www.bchotelandliquorconference.com/
For more information on the Alliance of Beverage Licensees, a non-profit organization funded by membership dues, our mission is to help your liquor or cannabis business succeed, visit: https://ablebc.ca/