Inventory Ledgers: Adjustments & Corrections
We recently announced the introduction of Inventory Ledgers in AirMed 5. This new functionality was designed to streamline your inventory management and recordkeeping. In this post we go into more details about the adjustments and corrections features in our inventory ledgers.
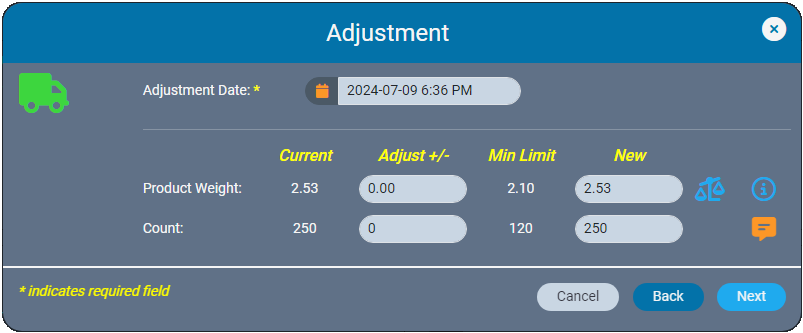
In the event of data entry errors, inventory ledgers make it easy to adjust weights and counts to resolve discrepancies. AirMed ensures that backdated adjustments are logically aligned with all prior and subsequent records. The ledger prevents negative balances by automatically validating that backdated weights or quantities are consistent with subsequent records.

Whether you are making a correction immediately or at a later date, AirMed offers multiple correction tools. Previously found in the Actions Menu, these options are now located in the ‘Admin Actions’ section, visible only to users with supervisor-level access.
For example, if you create a new inventory item with an incorrect date, you can amend the creation date based on the date of the parent record. All changes are logged in both the affected item’s ledger and the parent record ledger.
Additionally, new options are available to delete incorrect records or merge materials back into their original source when appropriate.
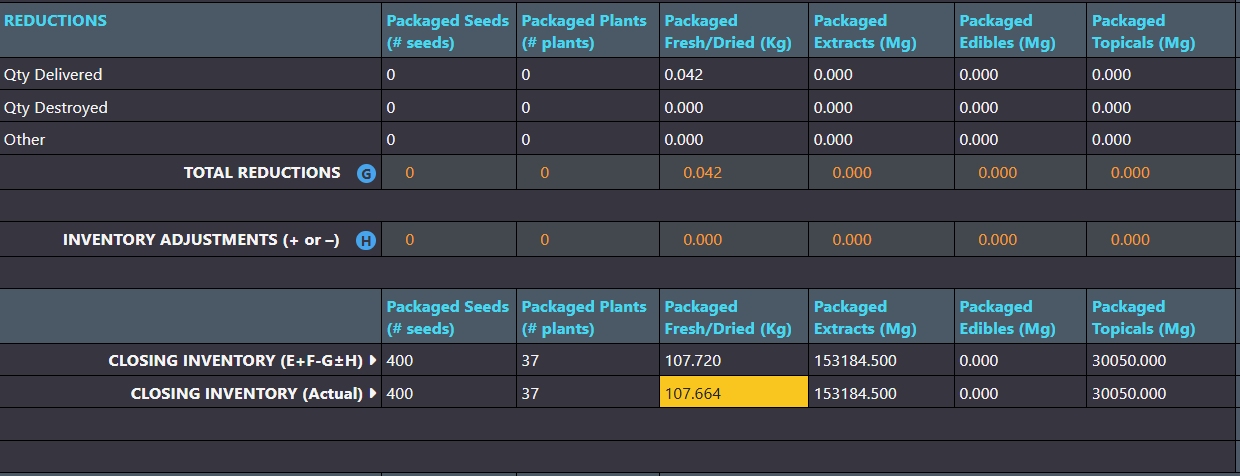
Adjustments that affect inventory levels are clearly categorized in monthly compliance reports.
While mistakes are inevitable, you can leverage AirMed’s technology to get you back on track.
We designed our new inventory ledgers to empower you to correct errors and ensure that your records accurately reflect your physical inventory. For more information about AirMed 5 visit our Software page.
Inventory Ledgers: Intelligent Backdating
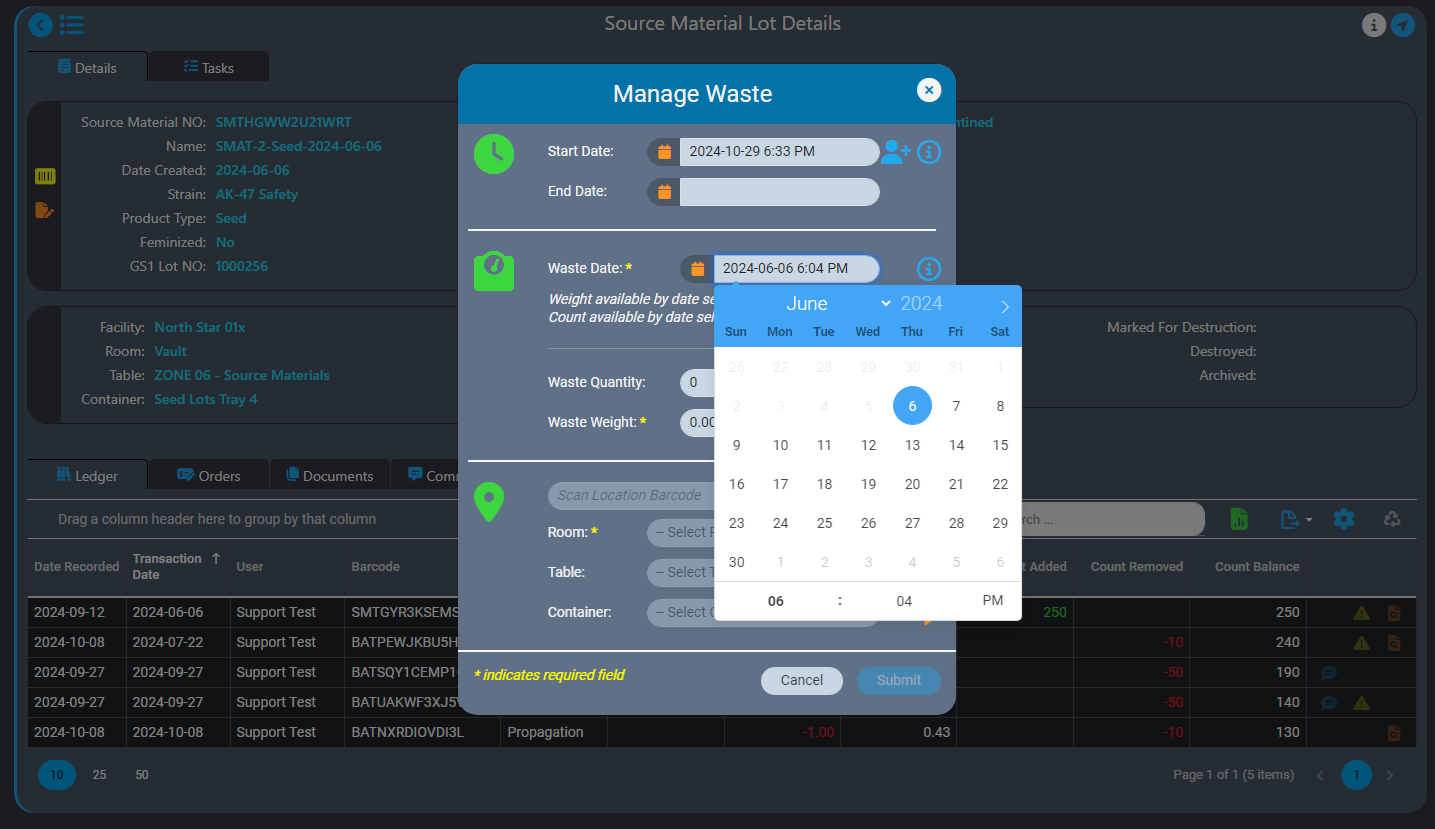
We recently announced the introduction of Inventory Ledgers in AirMed 5. In this post we go into more details about the intelligent backdating feature in our inventory ledgers.
Our intelligent record backdating ensures that any quantity or weight you enter will not lead to discrepancies in subsequent records. When backdating an adjustment, AirMed checks that it aligns logically with all successive records.
For instance, if you need to log waste for a source material, our safeguards won’t let you accidentally backdate a record to a date that is before the source material was created. You can only enter a date that is after the date the source material was created.
If an inventory item is created with the wrong date, admin tools can be used to adjust the creation date to match the creation of the parent record (e.g., adjusting a lot’s creation date to align with the date of the harvest). All changes are logged in both the affected item’s ledger and the parent record’s ledger.
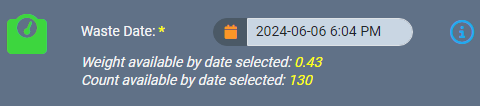
If a backdated action impacts monthly compliance reporting, AirMed will automatically generate an incident within our built-in Quality Management System (QMS). Supervisors can then investigate and resolve the incident, document the findings, and track it through a severity assessment and impact evaluation.
Having an efficient way of tracking and resolving discrepancies not only helps you meet compliance but also saves you time and money.
Intelligent backdating lets you reconcile your data with your physical inventory while satisfying your regulatory obligations. For more information about AirMed 5 visit our Software page.
Inventory Ledgers: Tracking
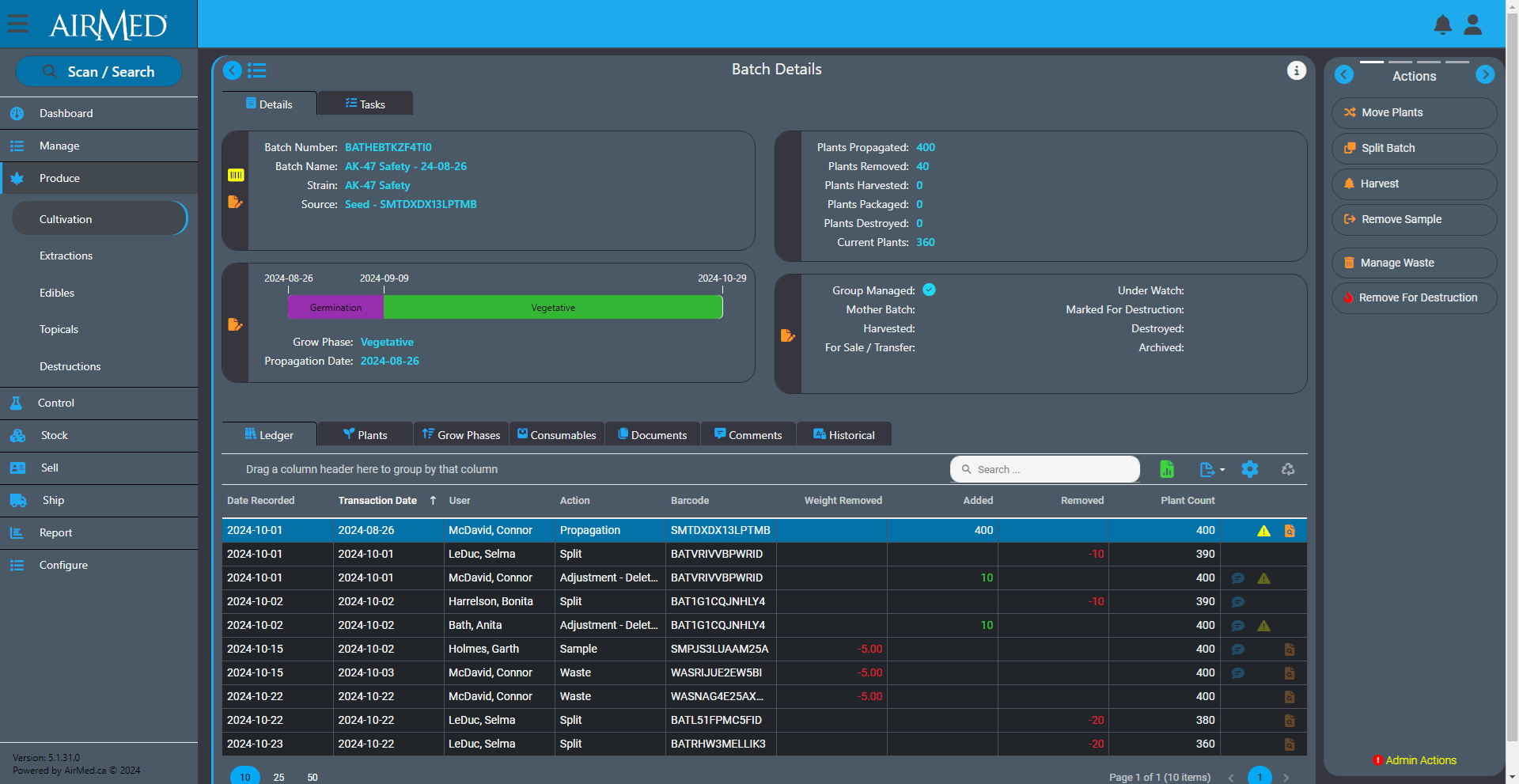
We recently announced the introduction of Inventory Ledgers in AirMed 5. This new functionality was designed to streamline your inventory management and recordkeeping.
The ledger lets you track actions performed on an inventory item. These actions are referred to as transactions in the ledger. You use the ledger as a perpetual register that records each transaction for an inventory item.
To use the ledger for a batch, for example, open the Batch Details screen and look for the Ledger tab. The tab shows a table that you use to record individual transactions related to that batch.

Each row offers fields for the date that the transaction took place along with the date the transaction was recorded in case they are different. There are also fields for the type of action that occurred, the user who performed the action and weights or counts added or removed during the action. The final column provides icons to visibly indicate adjustments or corrections to the batch. You can re-order the columns in the table by dragging.
The benefits of inventory ledgers are seen in both operational efficiency and compliance. Not only do they let you monitor the progress of a batch or lot, but tracked actions can be used to create a template for future production. During an audit, the ledger serves as a detailed register about each item, which can be used to validate processes and verify physical inventory.
Our inventory ledgers are designed to help you optimize your operations and ultimately enhance profitability. For more information about AirMed 5 visit our Software page.
MJBizCon 2024: Dec 3-6 in Las Vegas

The 13th annual MJBizCon is taking place December 3-6 in Las Vegas. Billed as the ultimate hub where global cannabis executives come together, this event is for all industry members from cultivators and manufacturers to retailers and brands to the essential suppliers that serve the community.
“MJBizCon is where you FUEL YOUR FIRE. Ignite your passion, spark conversations, gain critical insights, and forge the business partnerships needed to achieve fire-status in the cannabis industry.”
This event hosted by MJBIz magazine will be held December 3 to 6, 2024 at the Las Vegas Convention Center. For more information visit: https://mjbizconference.com/
Report: Veterans and Cannabis Mental Health

The Mental Health Commission of Canada (MHCC) has published a report titled Closing Research Gaps on Cannabis and Mental Health – Veteran-Specific Findings.
Over the past five years, MHCC has led a research program to assess the impact of cannabis on the mental health of diverse populations. MHCC recent released the results of studies that explored the relationship between cannabis and mental health among Canadian veterans.
“According to these studies, Canadian Veterans are significantly impacted by a range of physical and mental health conditions, which medical cannabis is frequently used to manage.”
Key findings in this report include the following.
- Veterans use cannabis for many reasons and experience a range of benefits and harms.
- The relationship between cannabis use and trauma is unique and nuanced for veterans.
- Barriers to accessing medical cannabis persist after legalization.
- Stigma prevents many veterans from accessing formal health-care supports.
The Mental Health Commission of Canada is a national non-profit organization created by the Canadian government in 2007 in response to a senate committee tasked to study mental health, mental illness, and addiction.
To read the full report, visit:
https://mentalhealthcommission.ca/resource/cannabis-synthesis-report-veterans/
Business of Cannabis Summit 2024: Nov 26 in Berlin

Since 2017 Business of Cannabis has highlighted the companies, brands, people and trends driving the industry. The Berlin Executive Summit will connect, inspire, and empower top cannabis industry leaders from Germany and across Europe. Dive into groundbreaking discussions, innovative strategies, and forward-thinking practices that will shape the future of Germany’s dynamic cannabis market. Don’t miss this unparalleled opportunity to elevate your leadership, drive growth, and forge powerful partnerships in the ever-evolving German and wider European cannabis landscapes.
Topics at this event include:
- Cannabis Associations
- Commercial Opportunities
- Home Cultivation
- Pillar 2 & Future Outlook
- Medical Cannabis
- Market Dynamics & Consolidation
This in-person event is being held November 26 at the PALISA, Palisadenstraße 48, 10243 in Berlin, Germany.
For more information visit: https://www.cannabisberlin.live/
AirMed 5 Introduces Inventory Ledgers

AirMed 5 now features powerful new functionality to simplify inventory management and improve recordkeeping. With the integration of inventory ledgers throughout the system, you can effortlessly track all transactions related to your inventory.
Comprehensive Tracking
Inventory ledgers provide detailed tracking of every addition or reduction to inventory, whether in batches or lots. Each entry includes the user responsible for the transaction, along with the date, time, transaction type, and the corresponding weight or quantity. This ensures full visibility into the usage of each batch and lot, providing a clear audit trail. Additionally, clickable links enable quick access to related records and associated comments.
Intelligent Backdating

Intelligent record backdating ensures that adjustments or entries made in the past do not cause discrepancies in future records or reporting. For example, if waste for a source material needs to be logged, AirMed ensures that the waste entry can be backdated only after the source material was created. The ledger also prevents negative balances by automatically validating that backdated weights or quantities are consistent with subsequent records. If a backdated action impacts monthly compliance reporting, AirMed will automatically generate an incident within our built-in Quality Management System (QMS).

Efficient Adjustments & Corrections

Inventory ledgers make it easy to adjust weights and counts to resolve discrepancies. AirMed ensures that backdated adjustments are logically aligned with all prior and subsequent records. Adjustments that affect inventory levels are clearly categorized as ‘other additions’ or ‘other reductions’ in monthly compliance reports.
Error Correction Options

In the event of data entry errors, AirMed offers multiple correction tools. For example, if an inventory item is created with the wrong date, admin tools can be used to correct the creation date to match the creation of the parent record (e.g., adjusting a lot’s creation date to align with the date of the harvest). All changes are logged in both the affected item’s ledger and the parent record’s ledger. Additionally, new options are available to delete incorrect records or merge materials back into their original source when appropriate.
The benefits of our new inventory ledgers include more accurate tracking, improved operational efficiency and easier compliance. For more information about AirMed 5 visit our Software page.
MedTech World Summit 2024: Nov 6-8 in Malta

The MedTech World Summit, which is reported to incorporate Medical Cannabiz World, is designed to connect money with medtech and bills itself as 2024’s leading MedTech startup & investor summit.
More than 3,000 attendees are expected from diverse regions including the UK, Europe, North America, Asia, the Middle East, and North Africa. This event promises networking meetups, investment opportunities with top MedTech investors, and connections with startups.
During the three-day event, attendees can participate in various activities such as panel discussions, CEO-only workshops, technology showcases, exhibitions, networking sessions, pitch competitions, cultural activities, as well as MedTech Malta Awards Gala.
This in-person event is being held November 6 through 8, 2024 in Valletta, Malta. For more information visit: https://med-tech.world/
Production Orders in AirMed 5
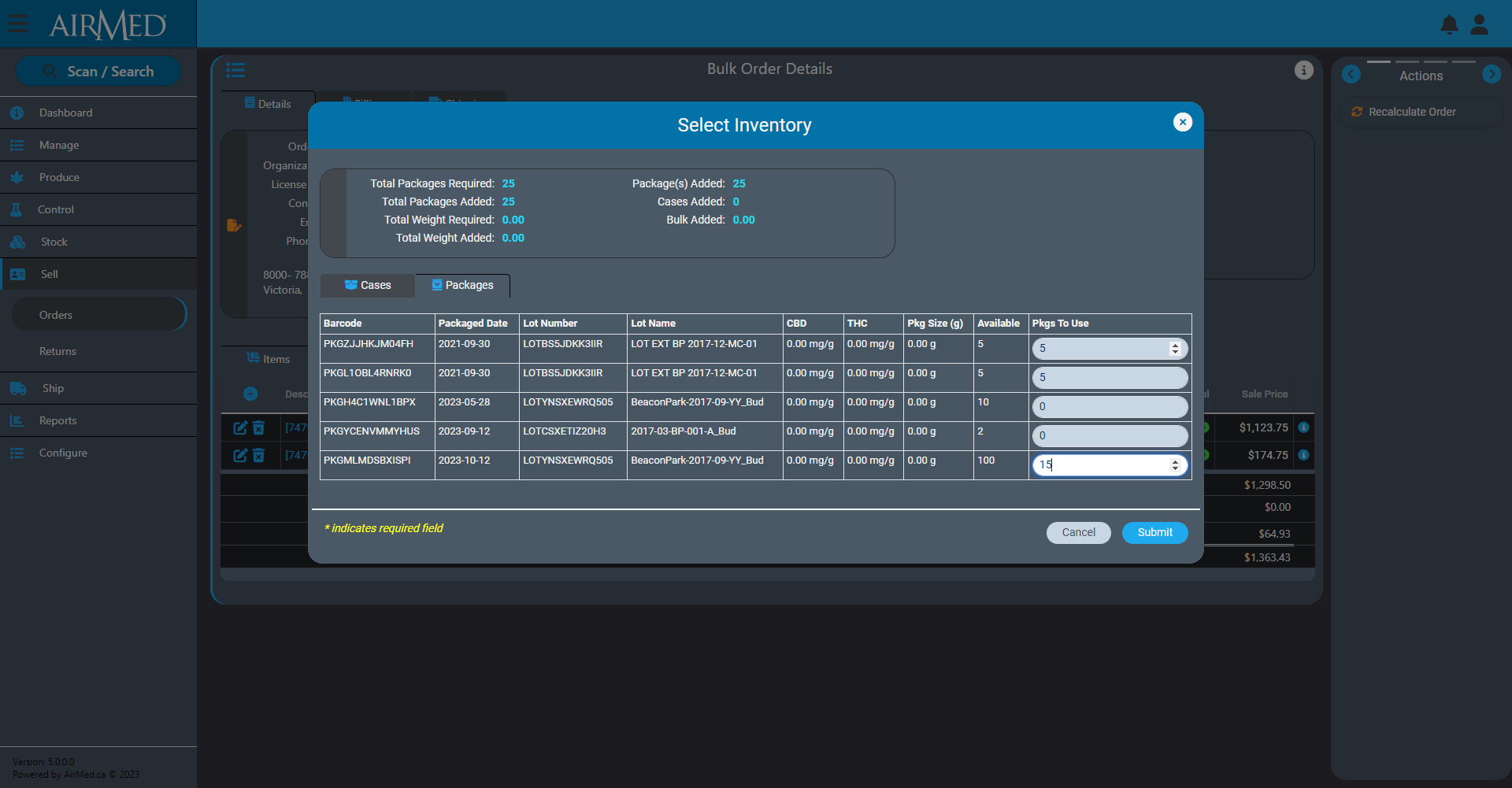
Our new Production Orders features will offer the ability to synchronize AirMed more easily with external enterprise resource planning (ERP) systems such as NetSuite or Microsoft Business Central.
When combined with tasks and approvals, this provides a high level of automation and ensures that product that is created meets market demand.
Internal Production Orders
Production Orders are essentially a wrapper for activities related to producing material such as cannabis, extractions, packages, topicals, or edibles.
Internal Production Orders are used to plan for production over a specified period of time. For instance, if producers are involved in cultivation, they can create internal production orders to produce new batches, harvest and dry them, and produce bulk products for sale or for additional processing.
Once a production order is created, it can optionally cascade out all the tasks related to the item being produced, even if some of the production requires routing to external organizations and suppliers.
Internal production orders can be used to plan production for any period of time. Multiple internal production orders can cover an entire year for a producer.
External Production Orders
External production orders are based on a sale to another organization, such as a provincial government distribution warehouse. An external production order would typically be tied to a purchase order from an external organization.
Using this feature, a producer will be able to see if there is existing inventory to fill an order. A producer can also create a new order that will require either growing and producing new cannabis or sourcing from another licensed producer.
Once the production order is approved (using the new Approval Workflow in AirMed), it will cascade out all related tasks to complete the order.
For instance, a producer makes a sale to a provincial distribution warehouse for 2000 vape cartridges. The producer can create a new batch of plants as source material and then track the entire process from propagation to finished packages in master-cases loaded on a pallet to be shipped.
The entire production process can be tracked and reported on, even if the workflows to produce the vape cartridges require a third-party to manage the extraction and packaging stages.
For more information call or email us, fill out the request demo contact form or visit our Software page.
Scaling Craft Production Virtual Summit: Oct 23, 2024

The cultivation virtual summit by Grow Opportunity will investigate how to scale craft cannabis production in both an indoor and a greenhouse environment.
Join Grow Opportunity this Fall for an afternoon event taking place between 1 and 3:30 pm EDT, and hear what growers have to say about the challenges and triumphs attributed to scaling premium products. The virtual event consists of a keynote speaker, a panel discussion and Q&A opportunity with the speakers.
For more information visit: https://www.growopportunity.ca/virtual-events/focus-on-scaling-craft-production/
Report: Statista Cannabis Market Data & Analysis

The 2024 Cannabis Market Data & Analysis Report from Statista, a global data and business intelligence platform, provides an international oversight into the Cannabis market. This 72-page report offers insight into drivers, current market trends, key companies, consumer insights, and future developments in the market.
“New product innovation and a shift in attitude towards cannabis from consumers and governments have led to a growing Cannabis market. Overall, the Cannabis market realized a revenue of US$59.4 billion worldwide in 2023.”
The analysts at Statista predict that the cannabis market will grow at a CAGR of 3.01% between 2024 and 2029. “Particularly strong growth is expected in upper-middle-income countries, due to the growing trend of changes in cannabis legislation and the corresponding expansion of supporting infrastructure.”
Statista reports, “Together, the Recreational and Medical Cannabis segments account for 51% of the total global revenue, due to the major sales of cannabis for recreational and medical-use in Canada and the United States.”
Statista is a global data and business intelligence platform with an extensive collection of statistics, reports, and insights on over 80,000 topics from 22,500 sources in 170 industries. Established in Germany in 2007, Statista operates in 13 locations worldwide and employs around 1,100 professionals.
For more information about this report visit: https://www.statista.com/study/119812/cannabis-market-data-and-analysis/
Canadian Greenhouse Conference 2024: Oct 9-10 in Niagara Falls

The Canadian Greenhouse Conference is Canada’s foremost event for commercial greenhouse flower, vegetable, cannabis, berry and nursery growers. This event is the ultimate connection point for growers, suppliers and research partners.
The Canadian Greenhouse Conference is a not-for-profit corporation and Canada’s foremost event and connection point for commercial growers of crops produced in a controlled environment. The conference attracts growers from across North America, gathers experts from around the world and showcases innovative production techniques, research, products and technology.
Being held October 9-10 at the Niagara Falls Convention Centre, the Canadian Greenhouse Conference offers two days of informative, motivating sessions and a large trade show. For more information visit: https://www.canadiangreenhouseconference.com/
Global CanExec Summit 2024: Oct 3-4 in Toronto

Held annually in Toronto, CANEXEC is a B2B Cannabis industry conference. CANEXEC is designed for Executives with leadership roles at leading cannabis producers.
This invitation-only event is attended by decision-makers from leading producers with 90+ percent of the speakers holding C-suite roles.
This is an in-person event being held October 3-4, 2024 at The Omni King Edward Hotel in Toronto, Canada.
For more information about the event, visit https://canexecsummit.com/
Introducing Our Updated WordPress Medical Plugin
Elevate Your Medical Cannabis Website Experience
We’re excited to announce the release of our enhanced WordPress Medical Plugin — now better than ever to help you build a cutting-edge medical cannabis website in Canada.
The AirMed Medical Plugin seamlessly integrates with Wordpress websites, letting licensed Canadian cannabis producers launch a fully functional medical cannabis platform. No need to upload product information to a third-party ecommerce site. Your brands and products feed directly onto your website from your AirMed database — in real time.
Our latest update, driven by customer feedback, introduces powerful new features designed to make your website stand out in a competitive market.
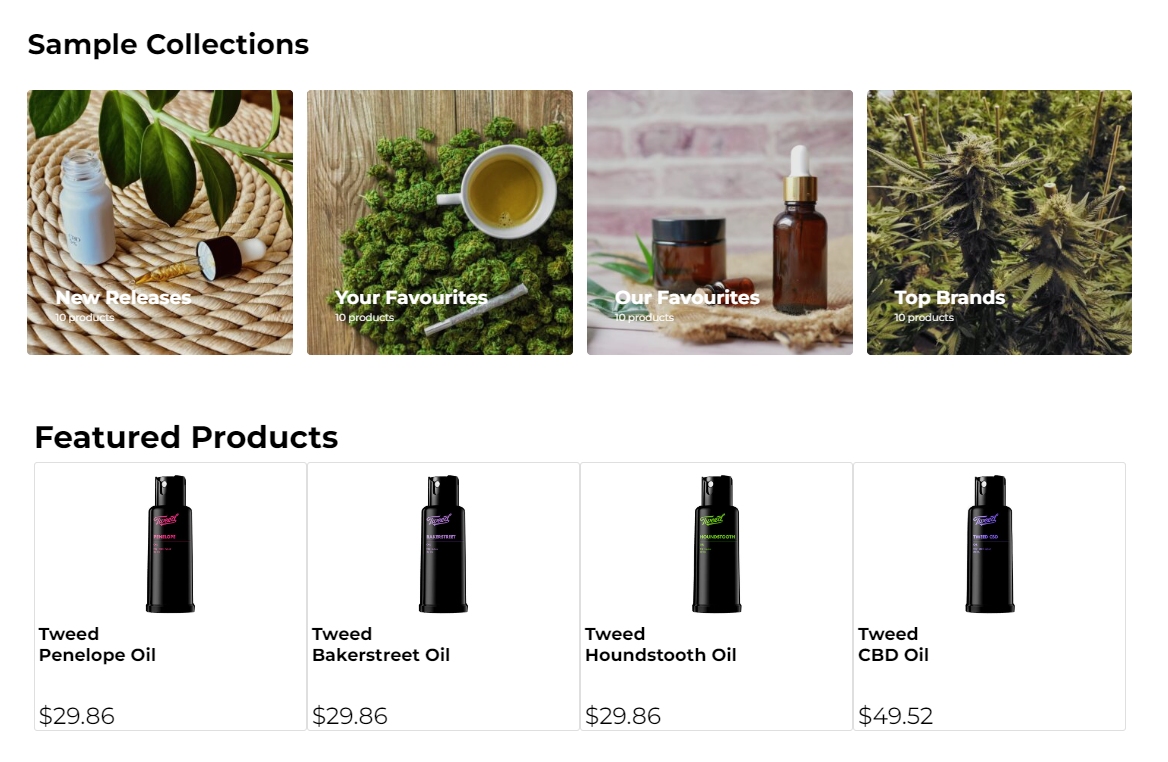
What’s New?
- Enhanced User Experience: We’ve added a sleek new carousel control, making it easier to showcase your products and services in a visually engaging way. Paired with optimized metatags, your site will not only look better but also attract more traffic through improved search engine visibility.
- Mobile-Friendly Design: Recognizing the importance of mobile users, we’ve updated all in-built webpages to deliver a seamless experience on smartphones and tablets. Your patients can now access crucial information, no matter where they are, with the same level of quality as on a desktop.
- Dynamic Dashboard Widgets: Stay on top of your site’s performance with our new dashboard widgets for summary pages. Get insights at a glance, making it easier than ever to manage and optimize your content.
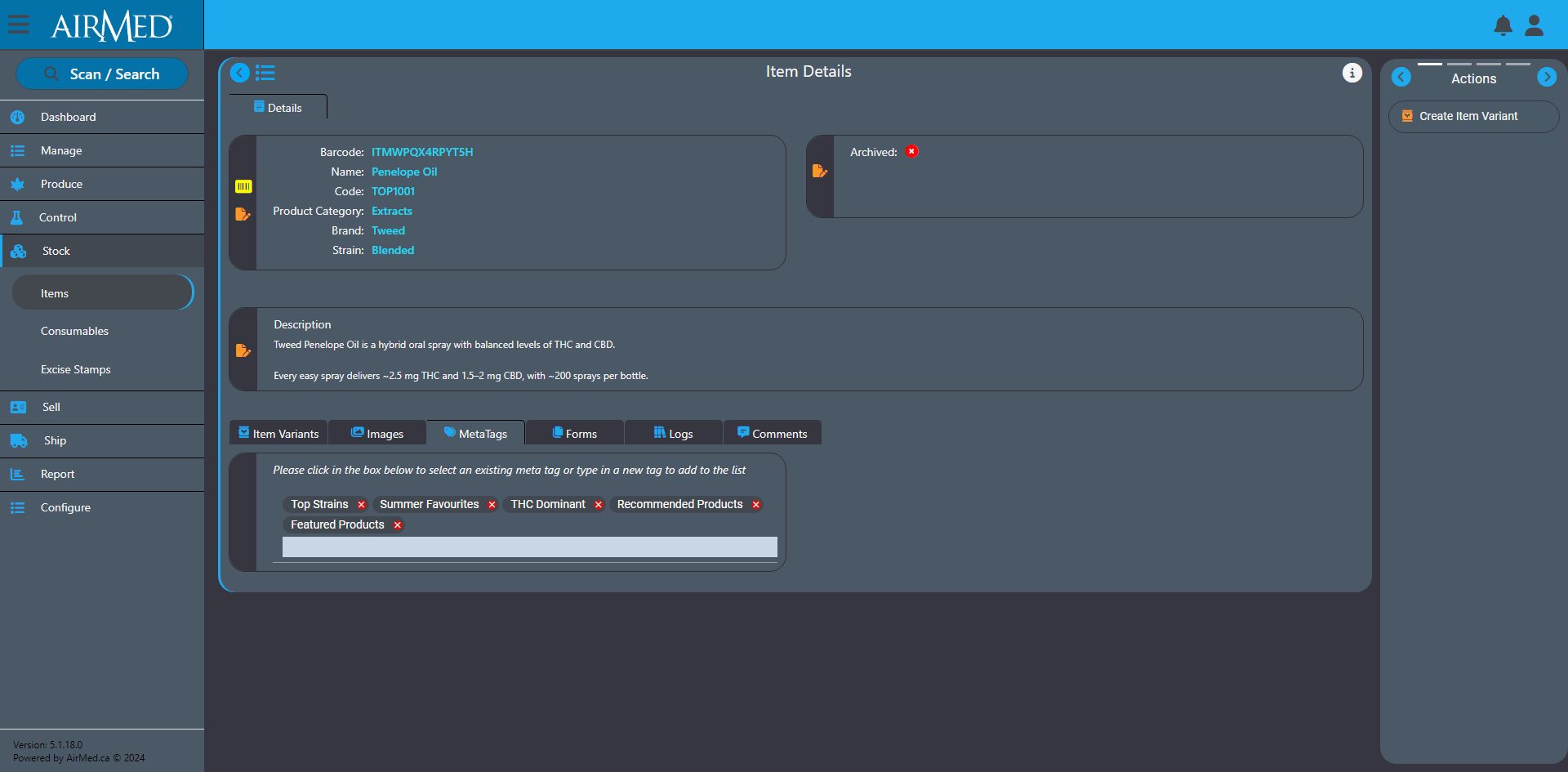
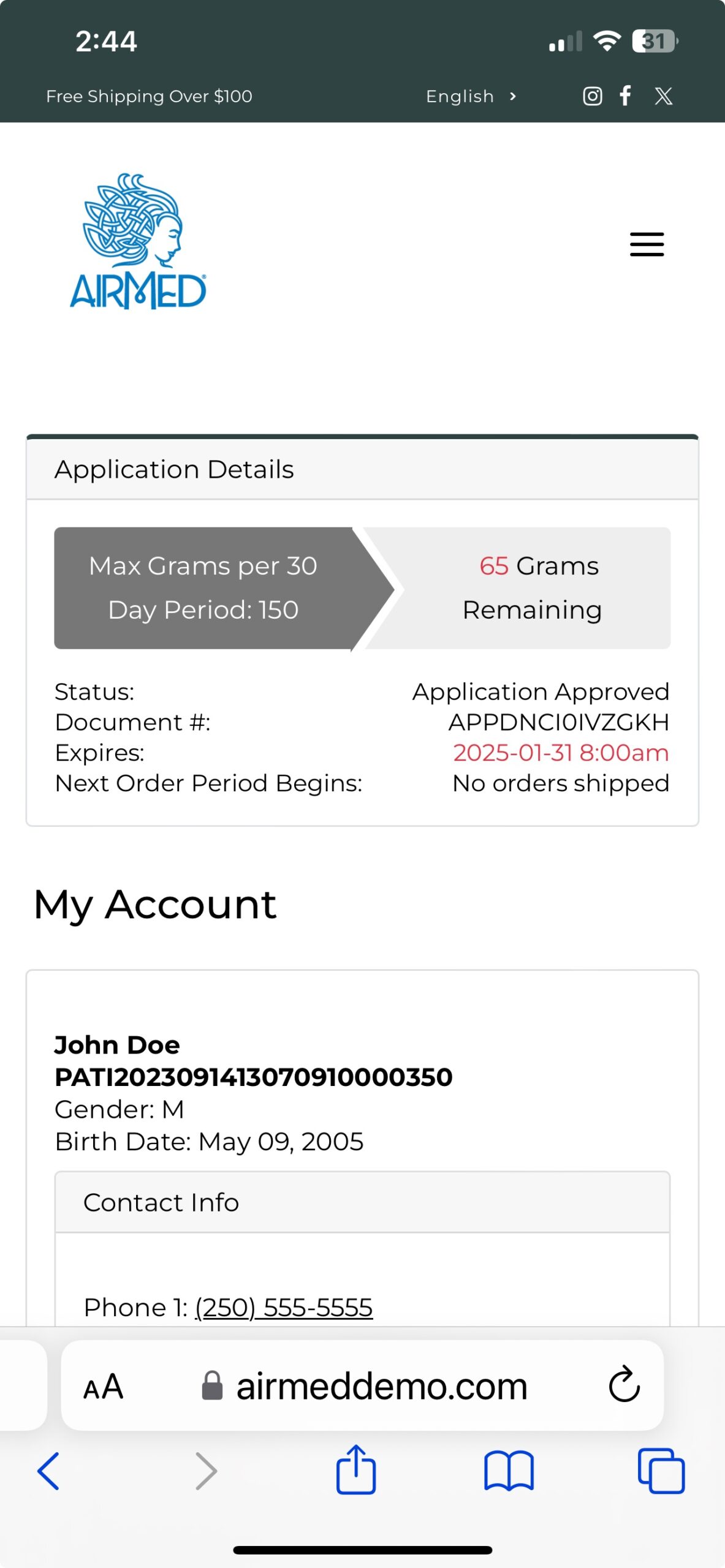
With our plugin, patients can register, submit applications, and purchase medical cannabis — with all data securely encrypted in full compliance of Health Canada regulations.
As an AirMed customer, you can quickly install the plugin and add a simple WordPress shortcode to display your product catalog.
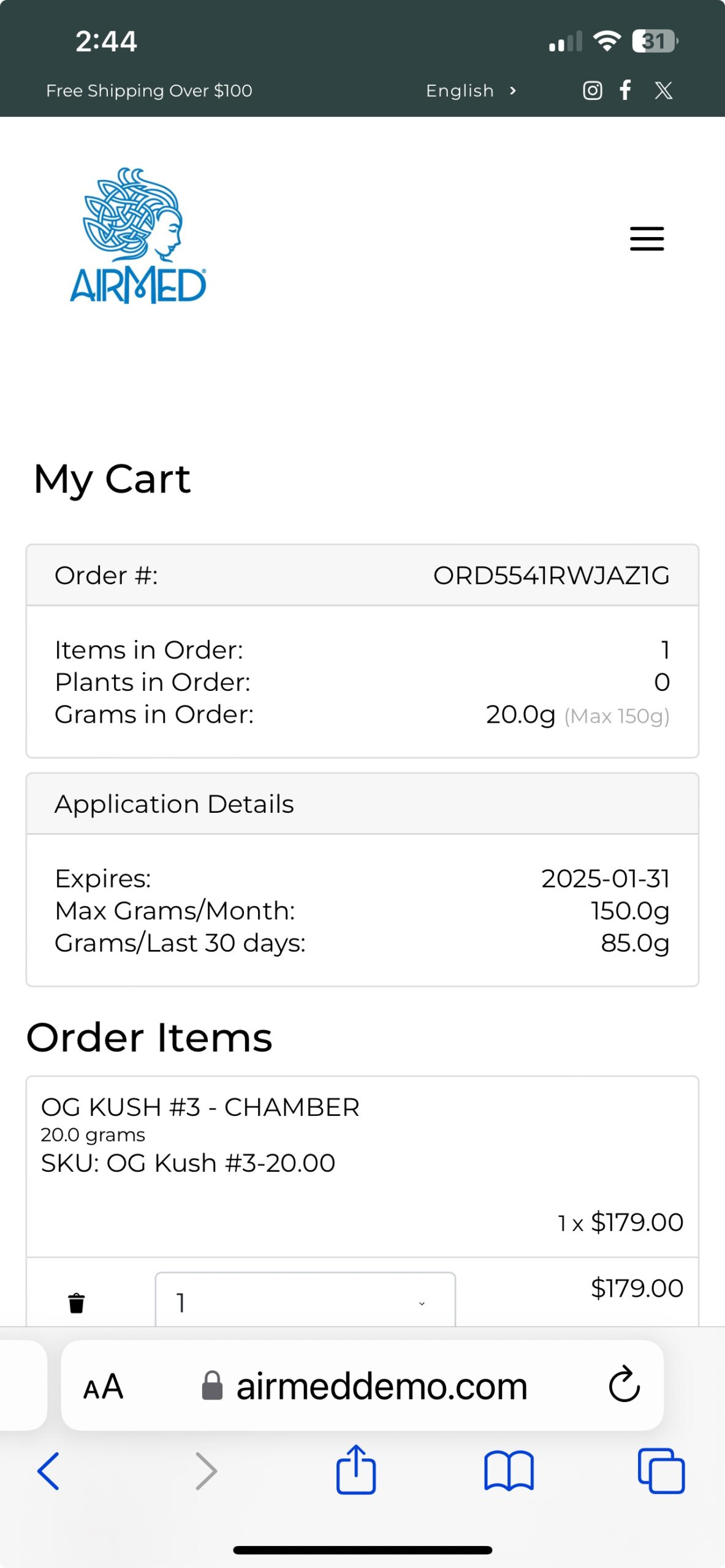
Whether you’re a clinic or medical cannabis producer, our updated WordPress Medical Plugin gives you the tools you need to create a compelling, user-friendly online presence.
Get started today and take your medical cannabis business to the next level!
Call us toll-free at 1-877-313-2442 to learn how our plugin can help you achieve your goals in the medical cannabis marketplace.
To see our plugin in action, visit our live demo site at airmeddemo.com.
Canadian Cannabis Leadership Summit: Oct 1 in Ottawa

Join the Cannabis Council of Canada for an afternoon of insightful discussion about the top issues facing the Canadian cannabis industry.
Bringing together leaders from the public and private sectors, as well as experts in medical research and financial services, this event will collectively advance issues which represent a risk to a properly functioning sector. Panel topics include in-depth conversation about the excise duty, the excise stamp, as well as reducing the regulatory burden. Following the conference, ticket holders will have the opportunity to engage with panelists during a private reception, providing a unique chance to network and deepen discussions on the most pressing issues. Secure your place at this event and ensure your voice is part of the conversation as we drive the Canadian cannabis industry forward.
Hosted in the O’Born Room at the National Arts Center (NAC) in Ottawa, just steps from Parliament Hill, this panel-style conference will run from 1 PM to 5 PM on Tuesday, October 1, 2024. For more information visit: https://cannabis-council.ca/events
Grow Up Alberta 2024 Conference: Sep 28-Oct 1 in Edmonton

From the latest in technology to workshops with the brightest minds in the industry, combined with the opportunity to sell your brands, Grow Up Conference and Expo is truly all-in-one stop event for the whole team.
Learn from the best at Masterclass Technical Workshops. Share thoughts, learn and network at the Growers Luncheon. Get expert tips on trimming methods and packaging. Explore the expo floor for the latest in technology or to meet with current suppliers.
Grow Up Alberta 2024 will have the largest collection of retail and wholesale buyers in Canada. your product in front of the people that make the buying decisions. Flower, concentrates, extracts, edibles, beverages and topicals will be on display and ready for purchase orders to be made.
For more information visit: https://growupconference.com/
Cannabis & GMP/GACP: Part 7 - Options

Welcome to our summer series on cannabis and GMP/GACP. A new article will be published once a week throughout the summer. You can access related articles that have been published so far by clicking the Compliance category on the main News & Events index page: Compliance category
As mentioned in earlier posts, any software used for Good Manufacturing Practice-related activities must be validated in the facility at the time of certification. Even if a software system has been validated in another facility in the past, it will need to be validated in your facility if you are becoming Good Manufacturing Practice-certified.
But it isn’t just the cannabis management system that will need to be validated. Any software used in relation to compliance-related activities or data must be validated at the time of GMP certification.
In addition, every piece of equipment must also be validated for GMP. Since most equipment today relies heavily on software for its operation, all of that software will need to validated along with the equipment.
The process is not only time-consuming but extremely costly. Some organizations have reported spending up to two years with costs into six figures on the GMP certification process.
Is there another option? Yes.
If Canadian LPs wish to sell cannabis in Europe or other GMP regions, there are two options.
a) Become Good Manufacturing Practice certified OR
b) Become Good Agricultural & Collection Practice certified and sell product in bulk to a producer who is GMP certified
There are pros and cons to both of these options.
While GMP certification lets producers sell their products directly to regions where it is required, as noted, the certification process involves validating every piece of equipment and software used in the facility. Plus, the cost for GMP certification is dependent on what products the licensed producer sells.
There is also a significant cost to maintaining a GMP certification including performing third-party vendor validations every two to three years as well as re-validating equipment and software when new releases/firmware updates are installed. These re-validation efforts require regression testing to perform complete process re-validation for all areas related to the specific equipment or software.
GACP certification does not permit producers to sell directly in GMP regions but does allow them to sell to GMP-certified producers. These certified producers can be in Canada or in the destination region.
Since GACP does not require third-party vendor validation nor equipment and software validation, time and costs to achieve certification are significantly reduced.
Whichever route you choose to take, if you use a cannabis management system in your facility, you should ensure that the software offers as many compliance-related features as possible.
If a software vendor claims their system is GMP-certified, it is not true. No software can become GMP-certified. A system can be validated in the GMP-certification process for a specific facility, but there is no GMP certification for software nor for equipment.
A software system having been validated in another facility that achieved GMP certification is an indication that it may meet compliance in your facility. But that is not a guarantee. If you are using software incorrectly or inconsistently, validation may fail.
And remember that all quality systems are heavily dependent on physical processes and practices. Software only supports the operation and supplies the recordkeeping and auditing. No software can make your facility GMP-compliant on its own.
Links
Following are links to the various standards that could be applied to cannabis.
Good Agricultural & Collection Practices/GACP (also called GAP): International; Israel
https://www.who.int/publications/i/item/9241546271
https://www.gov.il/en/departments/topics/medical-cannabis/govil-landing-page
Good Production Practices GPP: Canada
Good Manufacturing Practices/GMP: International; European Union; Australia
Current Good Production Practices/cGMP: USA
Good Distribution Practices/GDP: United Kingdom
https://www.gov.uk/guidance/good-manufacturing-practice-and-good-distribution-practice
Cannabis & GMP/GACP: Part 6 - Software Validation

Welcome to our summer series on cannabis and GMP/GACP. A new article will be published once a week throughout the summer. You can access related articles that have been published so far by clicking the Compliance category on the main News & Events index page: Compliance category
Validated Software vs GMP-ready Software
As noted, there is no certification for software. Software can only be validated.
For facilities seeking GMP certification, there are two types of software validation: system validation and process validation.
System validation involves a review of the software against the criteria for computerized systems in the quality system. The vendor must be qualified, along with their internal processes, to ensure that the software has been developed in a way that meets good practices for privacy, security, data integrity and more.
Process validation involves running tests of critical operations to ensure that the software performs as specified and that the results meet the guidelines.
These validations happen during the facility certification process.
Most computerized system vendors offer validation support to help their clients through the certification process. This support may include test scripts and documentation.
But be aware that just because software has been validated does not mean that it covers all GMP requirements. In fact, software that has been validated could have only one GMP-related feature, but that one feature was reviewed during the certification of a facility.
What validation does is ensure that the software meets the criteria for computerized systems and that any features that manage GMP-related processes are compliant.
Also be aware that any vendor can claim that their software is GMP-ready, whether it has been validated or not. GMP-readiness implies that the software meets the criteria for computerized systems and that features in the software are compliant. But only on-site validation against the facility’s processes will determine if it meets compliance for certification.
And GMP-readiness, or even validation, is not an indication of how many GMP-related features one software has in comparison with another. Also know that GMP certification of a cannabis facility is not dependent on the software in use being pre-validated or claiming GMP-readiness. The proof is in the review of the software in relation to the processes in the facility.
Essentially, one software application should not be assumed better for GMP than any other software based on a claim of being GMP-ready or validated. The proof is in the functionality that each software system has in relation to GMP. And if a software vendor claims their system is GMP-certified or GACP-certified, know that this is untrue. Software cannot be certified, neither can any piece of equipment. Only a facility can be certified.
Validation Process
During GMP certification, all software applications that have GMP implications will need to be validated during certification.
The vendor will be scrutinized to ensure that they are a legitimate business and that they follow accepted practices in their software development. This typically requires a review of business licensing and standard operating procedures.
The software will also be tested against specific workflows to ensure that it functions as designed and as required to meet compliance. This typically involves utilizing product instructions and test scripts.
In the certification process, only GMP-related activities are validated. Activities outside the scope of GMP are not validated. For example, since growing and harvesting are not covered by GMP, the facility would not validate those during the certification process. An LP selling dried cannabis would start the validation process at the drying stage and move on through testing, packaging, labelling, and distribution.
Plus, activities outside the scope of the facility are also not validated. If a facility is not manufacturing edibles, the GMP section that covers that type of product would not be included in the certification process.
Each certification process is unique to the facility and to the products being manufactured.
Links
Following are links to the various standards that could be applied to cannabis.
Good Agricultural & Collection Practices/GACP (also called GAP): International; Israel
https://www.who.int/publications/i/item/9241546271
https://www.gov.il/en/departments/topics/medical-cannabis/govil-landing-page
Good Production Practices GPP: Canada
Good Manufacturing Practices/GMP: International; European Union; Australia
Current Good Production Practices/cGMP: USA
Good Distribution Practices/GDP: United Kingdom
https://www.gov.uk/guidance/good-manufacturing-practice-and-good-distribution-practice
Cannabis & GMP/GACP: Part 5 - Cannabis Management Systems

Welcome to our summer series on cannabis and GMP/GACP. A new article will be published once a week throughout the summer. You can access related articles that have been published so far by clicking the Compliance category on the main News & Events index page: Compliance category
CMS Functionality
In terms of government regulations and industry standards, cannabis management software is an electronic records management system. Most business management software programs are essentially electronic records management systems regardless of what industry they are use in.
Since a computer record is only as secure as the system that holds it, the organization must therefore make certain that both the hardware and software comprising that system is secure. And since the hardware and software are only as secure as the facility they are located in, this also means securing the physical location of any servers or back-up copies of data and so on. If the hardware and software are stored elsewhere, such as in the case of cloud-based software, that location should be confirmed secure. As well, access to the system within the cannabis facility must also be secure. No computer system can meet such requirements—only an internal security policy and associated procedures can.
While we can’t stress enough that the physical practices are critical to meeting quality standards such as Good Manufacturing Practice (GMP) and Good Agricultural & Collection Practice (GACP), software can play a significant role by recording the activities of concern. A cannabis management system should meet the requirements for a computerized system, but the software should also offer all the functionality required for quality management and recordkeeping.
Following are the features to look for in a cannabis management system that will be used in a facility seeking GMP-certification.
Computerized System Requirements
- Maximum security and privacy of the hardware and software with a firewall and monitored intrusion system
- Encryption and security of sensitive data by both physical and electronic means against damage
- Stored data that is checked for accessibility, readability and accuracy with access ensured throughout the retention period
Controlled access to data based on worker roles and security profiles - Electronic signatures and multi-factor authentication to ensure only authorized employees access data and complete sensitive tasks
Good Production Practice
- A built-in quality management system with support for clearly defined standard operating procedures (SOPs)
- Recording of all production workflows carried out in the facility, which demonstrate that all the steps required by the defined procedures and instructions were in fact taken
- Sample management and recording of quality assurance testing with analysis results, including scanned copies of supporting documentation (e.g., certificate of analysis) if necessary
- Recording that the quantity and quality of the product was as expected
- Recording that no batch of product is released for sale or supply prior to certification by a Qualified Person that it is in accordance with the requirements of the relevant authorisations
- Recording of distribution that enables the complete history of a batch to be traced and retained in a comprehensible and accessible form
Deviations, Complaints & Recalls
- Recording of any significant deviations and investigations with the objective of determining the root cause and appropriate corrective and preventive action implemented
- Tracking of all pertinent information related to complaints, adverse reactions and severe adverse reactions
- A system for recording that complaints about products are examined, the causes of quality defects investigated and appropriate measures taken in respect of the defective products and to prevent reoccurrence
- An automated system to facilitate a recall of any batch of product from sale or supply
Waste & Destruction
- Tracking of waste material including where the waste was collected, by whom, the source of the waste (e.g., plant, batch, room, etc.), the weight, volume or count of the material and the current location of the waste item
- Recoding of destruction events including date, location, method, the authorized/qualified witnesses
Auditing & Reporting
- Complete audit trails for every action
- Product tracing to the lot or batch and ultimately back to the original genetic material
- Recording of the results of inspection and that testing of materials, intermediate, bulk, and finished products is formally assessed against specification
- Tracking of every interaction/communication with authorities, suppliers & clients
- Full compliance reporting, automated if possible to save time and facilitate audit success
- Controls to ensure the integrity of records throughout the retention period, with it clearly defined which record is related to each manufacturing activity and where this record is located
If your CMS offers all the above, not only will it help your business, but it will help make all compliance easier including GMP-certification.
Links
Following are links to the various standards that could be applied to cannabis.
Good Agricultural & Collection Practices/GACP (also called GAP): International; Israel
https://www.who.int/publications/i/item/9241546271
https://www.gov.il/en/departments/topics/medical-cannabis/govil-landing-page
Good Production Practices GPP: Canada
Good Manufacturing Practices/GMP: International; European Union; Australia
Current Good Production Practices/cGMP: USA
Good Distribution Practices/GDP: United Kingdom
https://www.gov.uk/guidance/good-manufacturing-practice-and-good-distribution-practice
Cannabis & GMP/GACP: Part 4 – Software Compliance

Welcome to our summer series on cannabis and GMP/GACP. A new article will be published once a week throughout the summer. You can access related articles that have been published so far by clicking the Compliance category on the main News & Events index page: Compliance category
Software & Quality Systems
Many of the principles in quality systems are dependent on physical practices, such as safety measures, security protocols, training, and carrying out activities in a prescribed and consistent manner.
The role of software in the scope of standards is to deliver the means for managing or tracking those practices and providing recordkeeping for auditing & compliance.
Please be aware that software cannot, on its own, become ‘certified’ in relation to GMP or GACP for that matter. Only a facility can become certified for either quality system. Software, however, must be ‘validated’ against specific practices carried out in the facility for GMP certification. As noted, GACP does not require software validation.
Each facility must validate their compliance-related processes during certification. In the case of GMP, the software and equipment used in the facility must also be validated. In the case of GACP, software and equipment are not validated – only processes.
Regardless of whether validation of equipment and software is required, certification is always dependent on more than software. Most of the principles and practices of quality systems are outside the control of any software application.
The Role of Software in Certification
For GMP, any software used for compliance-related activities must be validated in the facility at the time of certification. Even if a system has been validated in another facility in the past, the software will need to be validated in your facility.
Since quality systems cover so many aspects of the operation, it is possible that more than one software application may be used in a facility for compliance-related activities. The facility may have a cannabis management system to track production, but it may also have a separate quality management system or a laboratory management system. There may be a learning management system used to track worker qualifications plus a financial system that manages supplier-related data.
As all of these systems have compliance implications, each one, as well as each piece of related equipment, would need to be validated during certification, not just the cannabis management system.
In fact, a cannabis management system is not even mandatory for a cannabis facility seeking certification. A facility could be using several software systems that together manage all the various aspects of operations, none of which is a cannabis management system, and still become certified. Certification would involve having each application validated against the processes it covers.
However, a cannabis management system should make certification of any quality system easier, especially if it offers comprehensive tracking of all compliance-related processes as well as full auditing and reporting. Compliance functionality is one of the main selling points of cannabis management systems.
Links
Following are links to the various standards that could be applied to cannabis.
Good Agricultural & Collection Practices/GACP (also called GAP): International; Israel
https://www.who.int/publications/i/item/9241546271
https://www.gov.il/en/departments/topics/medical-cannabis/govil-landing-page
Good Production Practices GPP: Canada
Good Manufacturing Practices/GMP: International; European Union; Australia
Current Good Production Practices/cGMP: USA
Good Distribution Practices/GDP: United Kingdom
https://www.gov.uk/guidance/good-manufacturing-practice-and-good-distribution-practice