AirMed & QuickBooks
QuickBooks is a cloud-based accounting software suite designed for small to medium-sized businesses, offering tools for managing finances, tracking income and expenses, creating invoices, and handling payroll. QuickBooks from Intuit streamlines accounting tasks, automates workflows, and provides insights into business performance.
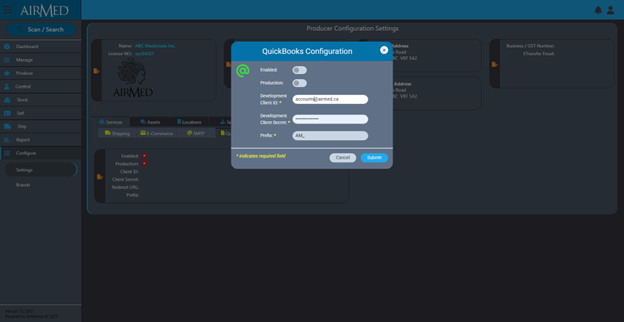
AirMed has added comprehensive integration with QuickBooks Online including full support for synchronizing AirMed Orders with QuickBooks Invoicing. This integration includes automatically creating item variants, customer organizations, as well as tax and term synchronization.
When you build an order in AirMed and release it to Production, you can automatically create an invoice in QuickBooks Online with all the same details. Modifications to orders in AirMed can automatically update QuickBooks with the corresponding changes.
The benefits of this kind of integration are numerous, especially for cannabis businesses navigating complex regulations and needing precise financial tracking.
Seed-to-Sale Tracking: AirMed excels at tracking products from cultivation to sale. Integrating this data with QuickBooks ensures that your financial records align perfectly with your inventory and operational data.
Eliminating Manual Data Entry: Our integration with QuickBooks not only saves significant time but also reduces the potential for human error that can occur with manual data entry.
Reporting & Audits: With both systems contributing to data management and audit trails, financial reporting is faster, and month-end closes are a breeze.
Real-time Financial Insights: AirMed already offered real-time data access and reporting, but with data flowing seamlessly to QuickBooks, you gain more visibility into your financial health.
If you are already familiar with QuickBooks, AirMed’s integration lets you leverage that familiarity, making it even easier to see your sales performance and profitability for faster, more informed decision-making.
For more information visit our Software page.
AirMed: Scalable Software for Your Cannabis Business

Last week we discussed building a scalable and sustainable business. Read the post here: Sustainably Scaling Your Cannabis Business.
Creating a cannabis business that is both scalable and sustainable is the best way to deal with an uncertain economy. One of the keys to scalability lies in having tools that can handle a varying volume of transactions, inventory, and data.
Our cannabis management software is designed with scalability in mind. Whether you’re a startup or a large enterprise, our platform grows with you.
Scaling up operations can mean more complex processes, such as adding new product lines, expanding to new locations, or hiring additional staff. AirMed lets you handle these complexities with ease. Features such as multi-location support, role-based access, and centralized reporting help you streamline processes and avoid bottlenecks.
AirMed manages every aspect of cultivation, processing, packaging, and distribution. We also offer quality management, workforce management, CRM and ERP-level functionality.
You can automate a series of tasks and assign them to different departments or users with activities cascading across the entire production cycle. Plan templates let you create formulas after completing the cultivation, drying and packaging of a successful batch. Our software automates inventory management and monitors stock levels in real-time to let you adjust to fluctuating demand.
With integrated tools for sales reporting, AirMed can provide data-driven insights to help you make smarter decisions. Whether it’s forecasting sales trends, identifying popular products, or tracking seasonal demand, data becomes even more valuable as the economy fluctuates.
Our system is module to let you add functionality if and when needed. We also offer flexible billing options, and we’re currently developing a lower-cost ‘lite’ version of AirMed to help micros. A limited feature set and simplified workflows will get your smaller operation up and running while ensuring that you meet compliance and stay within budget.
Investing in scalable software ensures that your business can handle changing demand, variable inventories, and flexible operations to set the stage for long-term success. With the right system in place, you can focus on your business, while knowing that your operations are efficient, compliant, and sustainable.
For more information visit our Software page or our Compliance page.
AirMed & Cannabis Inventory Management

Last week we discussed the benefits of inventory management. Read the post here.
This week, we’ll look at ways that AirMed helps streamline inventory management for cannabis businesses.
In 2024 we introduced ledgers designed to streamline your inventory management and recordkeeping.
AirMed integrates inventory ledgers throughout the system letting you effortlessly track all transactions related to your inventory. Our inventory ledgers provide detailed tracing of every addition or reduction in batches or lots with full audit trails. We also added intelligent backdating to ensure adjustments or entries made in the past won’t create discrepancies in the future. And we’ve made it easy for you to resolve inventory inconsistencies by offering multiple tools for making corrections.
But ledgers are not the only inventory management tools in AirMed.
Our software offers GS-1 barcoding with full functionality for multi-level packaging that lets you assemble retail-ready individual packages using a ‘master case’ system and identify & track products at each packaging level.
AirMed offers unparalleled ease while dealing with complex order requirements. Manage order requests, order planning & assembly, as well as shipping from one screen. After releasing a planned order to production, verify order items in one step. You can select available inventory from multiple lots for order fulfillment. Browse products, package sizes and available inventory, then initiate package runs and pack cases workflows in the items table.
Since AirMed reports on every item in the system, inventory reporting is available at the click of a button. Real-time inventory tracking lets businesses assess product demand more effectively, reducing excess inventory and lowering costs associated with unsold goods.
Utilizing cannabis inventory management is more than just a way to keep track of products—it’s a key to reducing losses, improving operational efficiency, and boosting profitability.
AirMed has been 100-percent Canadian owned and operated since it was created in 2014. Click the Request Demo button at the top of the page today to explore AirMed in a free walkthrough and learn what home-grown can do for you.
For more information about AirMed visit our Software page.
To read more about our inventory ledgers, read our previous posts.
AirMed 5 Introduces Inventory Ledgers
Still Proudly Canadian-Owned and Operated

After more than 10 years in business, we are incredibly proud to remain 100 percent Canadian-owned and operated. From day one, our mission has been clear: to provide world-class products and services while staying true to our Canadian roots.
Every decision we make is guided by our commitment to support the Canadian cannabis industry — from Cape Scott to Cape Spear and every point in between.
With hard work, innovation, and the continued loyalty of our customers, we’ve built something truly special and truly Canadian.
Through our second decade in business, we hope to continue serving you with the same passion, dedication, and Canadian pride that has defined us since 2014.
Take a tour of AirMed to see what homegrown can do for you. Call us today at (877) 313-2442 or use the Request Demo button at the top of the page.
In the meantime, visit our Software page.
Data-Driven Decision Making & AirMed
Data-driven decision-making is the practice of using facts, metrics, and data to guide strategic business decisions.
From optimizing cultivation processes to enhancing marketing strategies, the ability to analyze and act on data can make the difference between thriving and struggling in this dynamic cannabis space. By leveraging data, cannabis operators can gain actionable insights, reduce costs, improve yields, ensure compliance, and ultimately achieve a competitive edge.
Benefits of Data-driven Decision Making
Cannabis cultivation is a complex process influenced by numerous variables. By using data analytics, producers can monitor these variables in real-time, identify trends, and make adjustments to maximize yield and quality.
Cannabis is a resource-intensive product. Data-driven strategies help identify inefficiencies and optimize resource allocation. This ensures that resources are used as effectively as possible and reduces waste. These measures not only decrease operational expenses but also support sustainability initiatives—an important consideration for environmentally conscious consumers.
Compliance requires meticulous record-keeping and traceability throughout the supply chain. Data-driven systems that leverage information to demonstrate adherence to quality and safety standards enhance credibility with regulators and customers alike. Moreover, predictive analytics can help identify potential risks, enabling proactive measures to mitigate them.
As the cannabis industry matures, innovation is key to staying ahead of competitors. Data-driven insights can empower you to experiment with new cultivation techniques, product formulations, and market strategies. By staying informed and agile, you can seize opportunities and respond effectively to market shifts.
With narrow profit margins and significant operational costs, data-driven decision-making can also support financial sustainability. Data analytics can provide insights into cost drivers, helping you identify areas where they can reduce expenses or increase efficiency. Analyzing labor costs and production timelines might highlight opportunities to streamline operations, while inventory data can ensure that stock levels align with demand, reducing the risk of overproduction or stockouts.
AirMed & Your Data
AirMed has always gone beyond other systems by tracking and reporting thousands of fields of data.
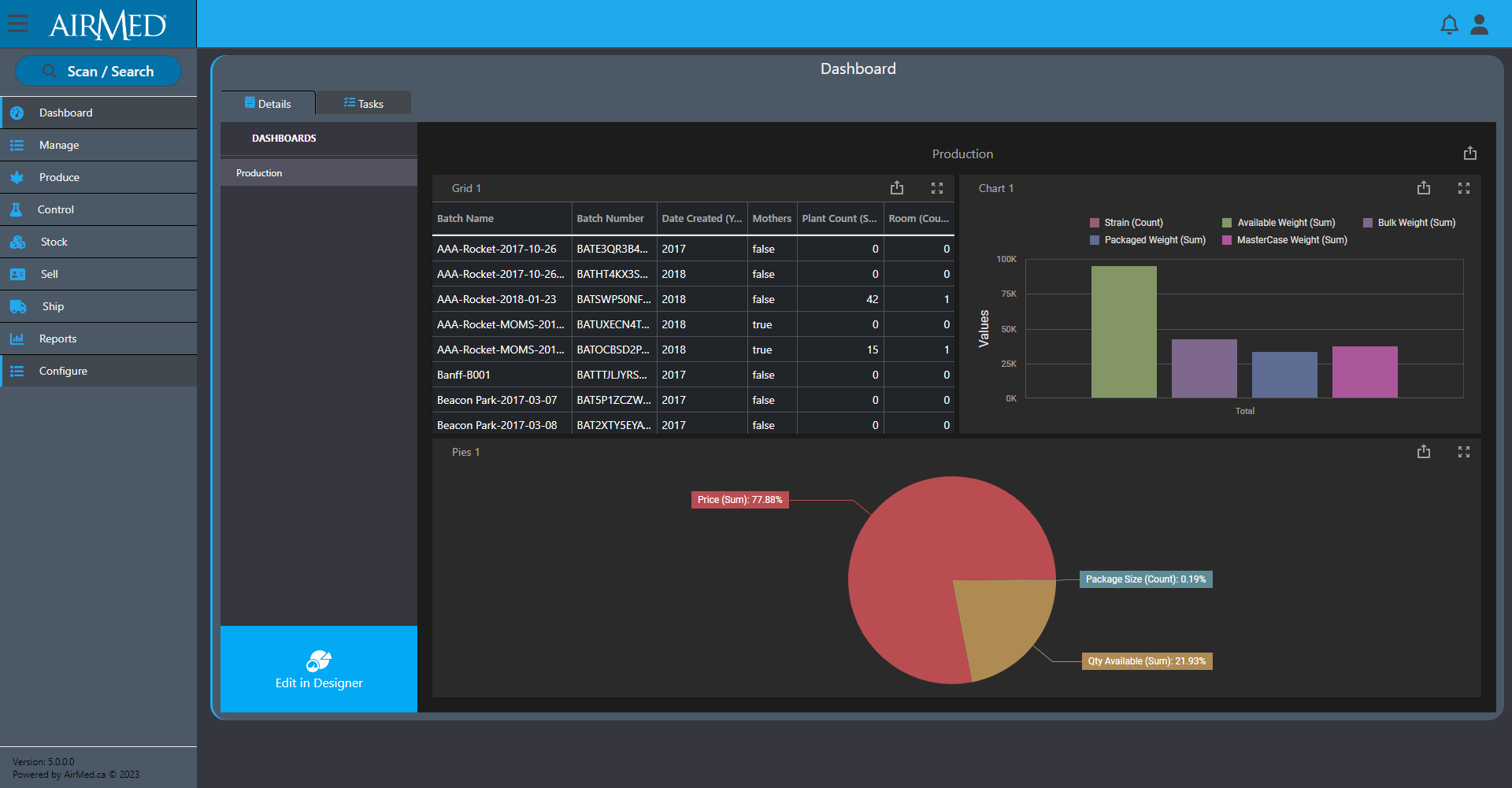
Dozens of reports come standard with AirMed, but we also offer an optional report designer to create unlimited custom reports with access to every field of data in the system. You can select individual fields to include in a report and add options to manage master-detail relationships, cross-tab reports, table and vertical reports, and filter options.
And our optional business intelligence (BI) designer provides pre-designed dashboard widgets that offer the best data visualization option for you. You can create insightful and information-rich decision support systems by simply selecting the appropriate UI widget: Chart, Pivot Table, Data Card, Gauge, TreeMap, Map, Grid, or simple Filter elements. By dropping data fields, results are immediate, accurate and always relevant.
For those who wish to edit or create new widgets, the BI Dashboard is engineered to let you spend more time on business and less on UI customization. Whether it’s manipulation of individual chart series, specifying a pivot table’s dimensions or connecting UI elements to fields across different data-sources or data providers, the BI Dashboard designer is built to make your experience a productive one.
Conclusion
Incorporating data-driven decision-making provides the ability to collect, analyze, and act on data to optimize processes, improve product quality, reduce costs, ensure compliance, and remain competitive. AirMed has been designed to help you embrace a data-driven approach.
For more information visit our Software page.
Inventory Ledgers: Adjustments & Corrections
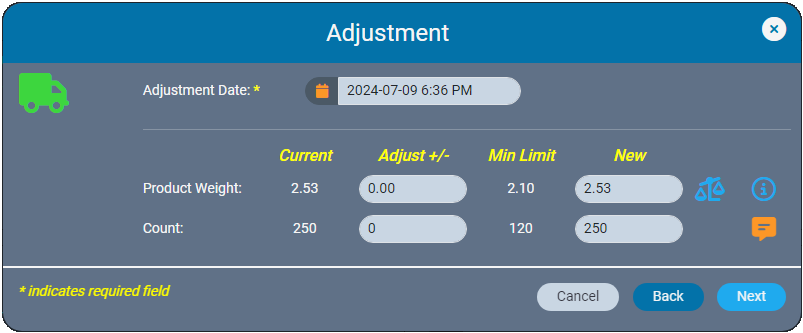
We recently announced the introduction of Inventory Ledgers in AirMed 5. This new functionality was designed to streamline your inventory management and recordkeeping. In this post we go into more details about the adjustments and corrections features in our inventory ledgers.
In the event of data entry errors, inventory ledgers make it easy to adjust weights and counts to resolve discrepancies. AirMed ensures that backdated adjustments are logically aligned with all prior and subsequent records. The ledger prevents negative balances by automatically validating that backdated weights or quantities are consistent with subsequent records.

Whether you are making a correction immediately or at a later date, AirMed offers multiple correction tools. Previously found in the Actions Menu, these options are now located in the ‘Admin Actions’ section, visible only to users with supervisor-level access.
For example, if you create a new inventory item with an incorrect date, you can amend the creation date based on the date of the parent record. All changes are logged in both the affected item’s ledger and the parent record ledger.
Additionally, new options are available to delete incorrect records or merge materials back into their original source when appropriate.
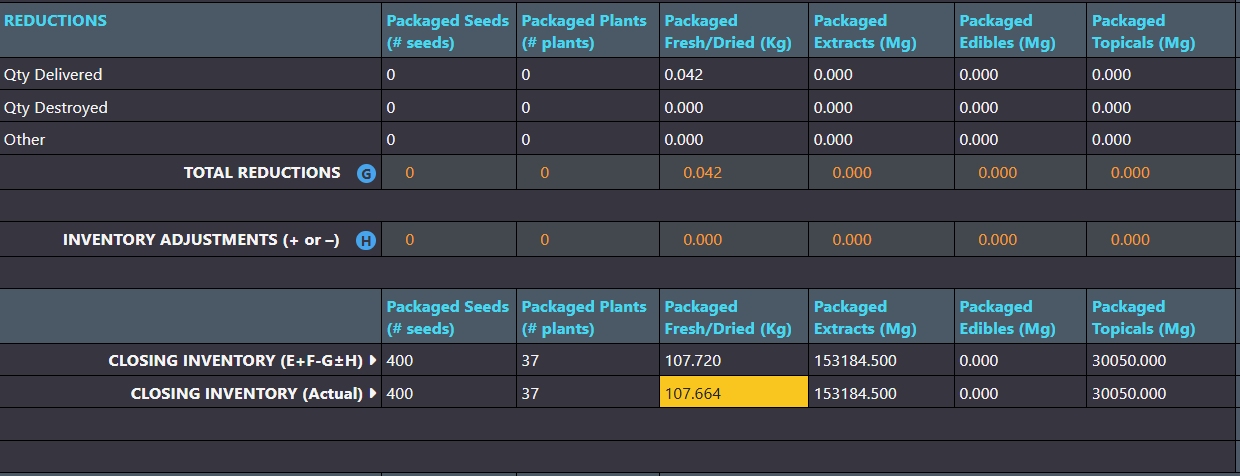
Adjustments that affect inventory levels are clearly categorized in monthly compliance reports.
While mistakes are inevitable, you can leverage AirMed’s technology to get you back on track.
We designed our new inventory ledgers to empower you to correct errors and ensure that your records accurately reflect your physical inventory. For more information about AirMed 5 visit our Software page.
Inventory Ledgers: Intelligent Backdating
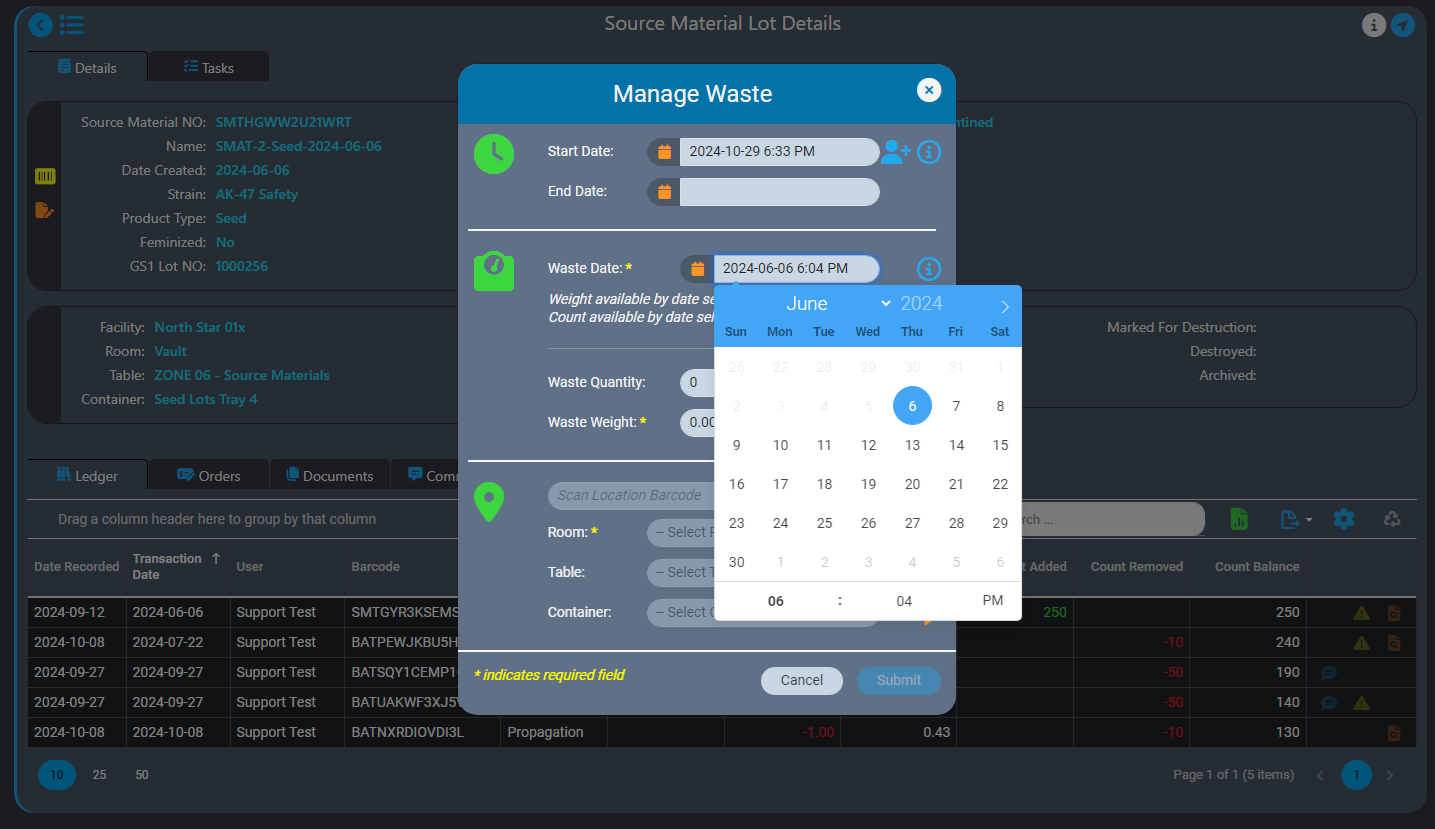
We recently announced the introduction of Inventory Ledgers in AirMed 5. In this post we go into more details about the intelligent backdating feature in our inventory ledgers.
Our intelligent record backdating ensures that any quantity or weight you enter will not lead to discrepancies in subsequent records. When backdating an adjustment, AirMed checks that it aligns logically with all successive records.
For instance, if you need to log waste for a source material, our safeguards won’t let you accidentally backdate a record to a date that is before the source material was created. You can only enter a date that is after the date the source material was created.
If an inventory item is created with the wrong date, admin tools can be used to adjust the creation date to match the creation of the parent record (e.g., adjusting a lot’s creation date to align with the date of the harvest). All changes are logged in both the affected item’s ledger and the parent record’s ledger.
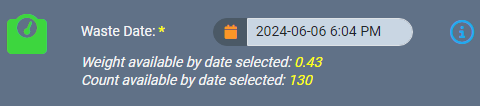
If a backdated action impacts monthly compliance reporting, AirMed will automatically generate an incident within our built-in Quality Management System (QMS). Supervisors can then investigate and resolve the incident, document the findings, and track it through a severity assessment and impact evaluation.
Having an efficient way of tracking and resolving discrepancies not only helps you meet compliance but also saves you time and money.
Intelligent backdating lets you reconcile your data with your physical inventory while satisfying your regulatory obligations. For more information about AirMed 5 visit our Software page.
Inventory Ledgers: Tracking
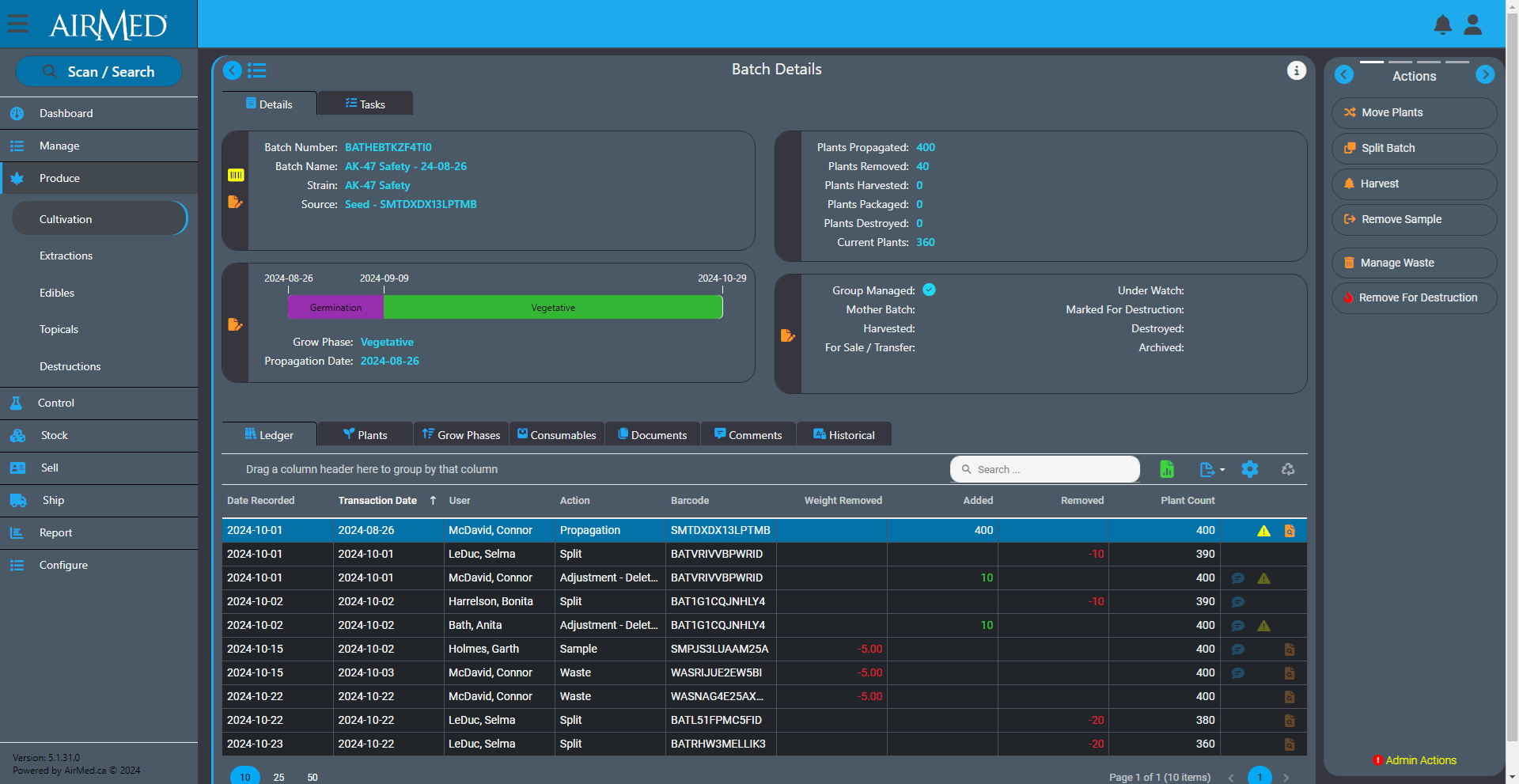
We recently announced the introduction of Inventory Ledgers in AirMed 5. This new functionality was designed to streamline your inventory management and recordkeeping.
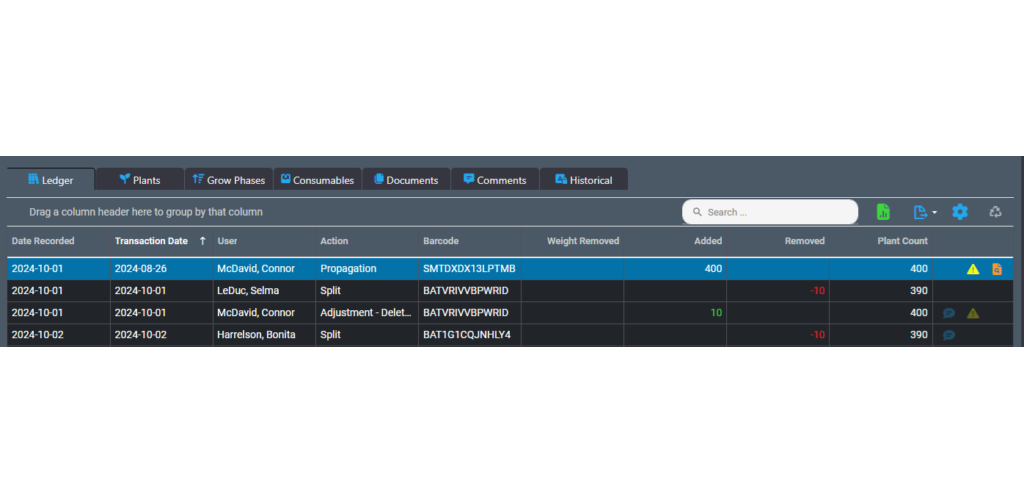
The ledger lets you track actions performed on an inventory item. These actions are referred to as transactions in the ledger. You use the ledger as a perpetual register that records each transaction for an inventory item.
To use the ledger for a batch, for example, open the Batch Details screen and look for the Ledger tab. The tab shows a table that you use to record individual transactions related to that batch.

Each row offers fields for the date that the transaction took place along with the date the transaction was recorded in case they are different. There are also fields for the type of action that occurred, the user who performed the action and weights or counts added or removed during the action. The final column provides icons to visibly indicate adjustments or corrections to the batch. You can re-order the columns in the table by dragging.
The benefits of inventory ledgers are seen in both operational efficiency and compliance. Not only do they let you monitor the progress of a batch or lot, but tracked actions can be used to create a template for future production. During an audit, the ledger serves as a detailed register about each item, which can be used to validate processes and verify physical inventory.
Our inventory ledgers are designed to help you optimize your operations and ultimately enhance profitability. For more information about AirMed 5 visit our Software page.
AirMed 5 Introduces Inventory Ledgers

AirMed 5 now features powerful new functionality to simplify inventory management and improve recordkeeping. With the integration of inventory ledgers throughout the system, you can effortlessly track all transactions related to your inventory.
Comprehensive Tracking
Inventory ledgers provide detailed tracking of every addition or reduction to inventory, whether in batches or lots. Each entry includes the user responsible for the transaction, along with the date, time, transaction type, and the corresponding weight or quantity. This ensures full visibility into the usage of each batch and lot, providing a clear audit trail. Additionally, clickable links enable quick access to related records and associated comments.
Intelligent Backdating

Intelligent record backdating ensures that adjustments or entries made in the past do not cause discrepancies in future records or reporting. For example, if waste for a source material needs to be logged, AirMed ensures that the waste entry can be backdated only after the source material was created. The ledger also prevents negative balances by automatically validating that backdated weights or quantities are consistent with subsequent records. If a backdated action impacts monthly compliance reporting, AirMed will automatically generate an incident within our built-in Quality Management System (QMS).

Efficient Adjustments & Corrections

Inventory ledgers make it easy to adjust weights and counts to resolve discrepancies. AirMed ensures that backdated adjustments are logically aligned with all prior and subsequent records. Adjustments that affect inventory levels are clearly categorized as ‘other additions’ or ‘other reductions’ in monthly compliance reports.
Error Correction Options

In the event of data entry errors, AirMed offers multiple correction tools. For example, if an inventory item is created with the wrong date, admin tools can be used to correct the creation date to match the creation of the parent record (e.g., adjusting a lot’s creation date to align with the date of the harvest). All changes are logged in both the affected item’s ledger and the parent record’s ledger. Additionally, new options are available to delete incorrect records or merge materials back into their original source when appropriate.
The benefits of our new inventory ledgers include more accurate tracking, improved operational efficiency and easier compliance. For more information about AirMed 5 visit our Software page.
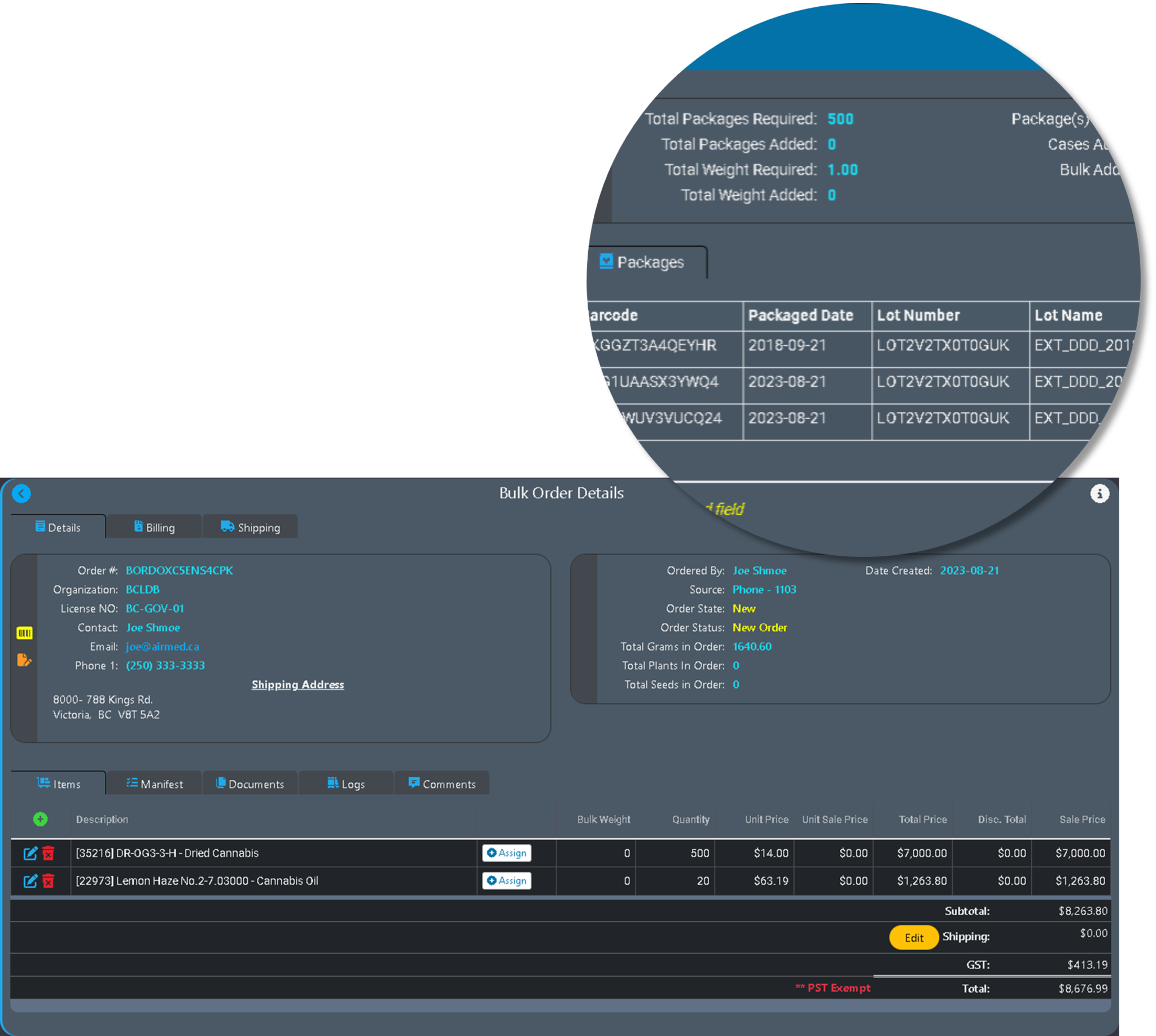
Production Orders in AirMed 5
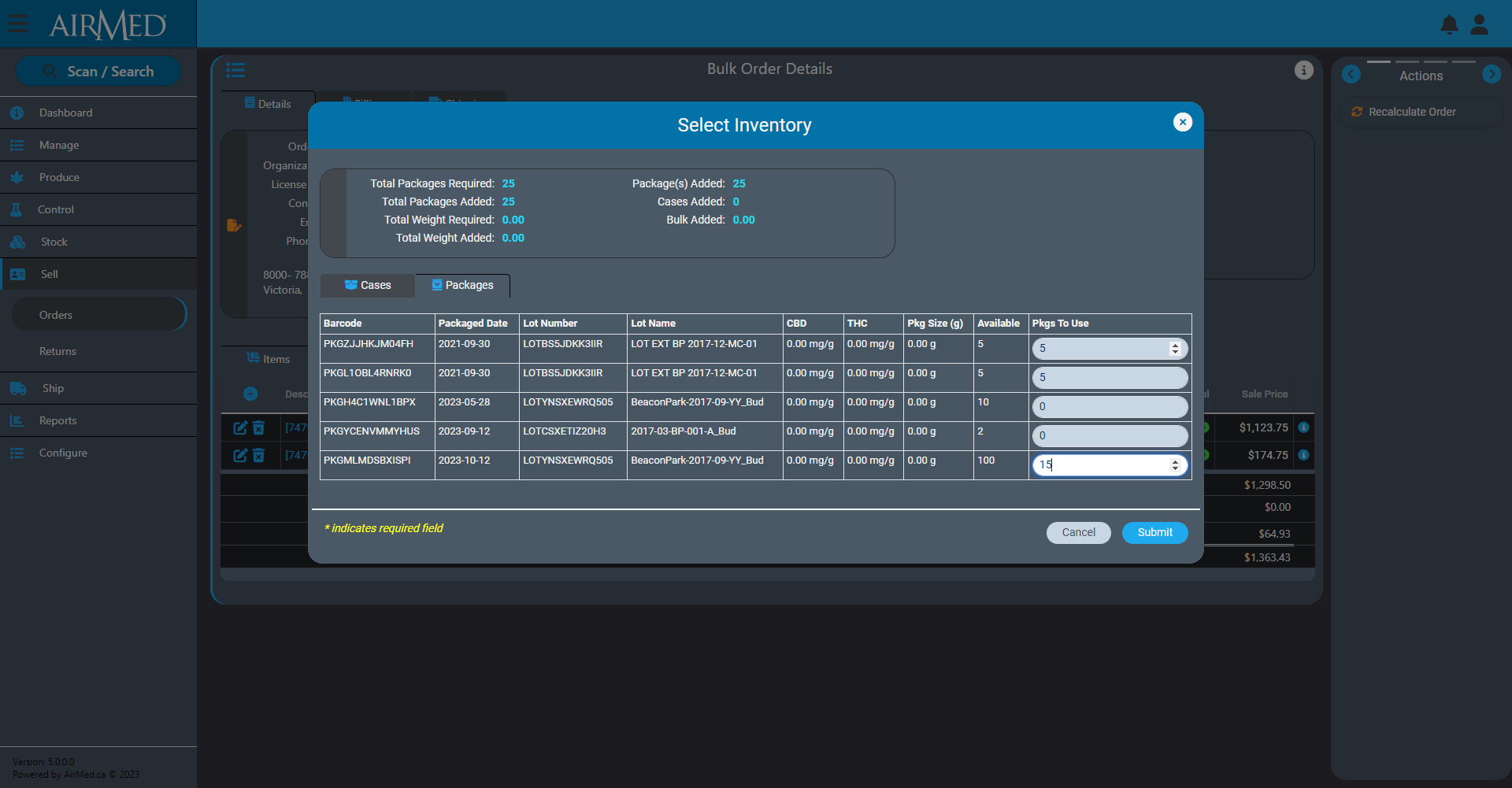
Our new Production Orders features will offer the ability to synchronize AirMed more easily with external enterprise resource planning (ERP) systems such as NetSuite or Microsoft Business Central.
When combined with tasks and approvals, this provides a high level of automation and ensures that product that is created meets market demand.
Internal Production Orders
Production Orders are essentially a wrapper for activities related to producing material such as cannabis, extractions, packages, topicals, or edibles.
Internal Production Orders are used to plan for production over a specified period of time. For instance, if producers are involved in cultivation, they can create internal production orders to produce new batches, harvest and dry them, and produce bulk products for sale or for additional processing.
Once a production order is created, it can optionally cascade out all the tasks related to the item being produced, even if some of the production requires routing to external organizations and suppliers.
Internal production orders can be used to plan production for any period of time. Multiple internal production orders can cover an entire year for a producer.
External Production Orders
External production orders are based on a sale to another organization, such as a provincial government distribution warehouse. An external production order would typically be tied to a purchase order from an external organization.
Using this feature, a producer will be able to see if there is existing inventory to fill an order. A producer can also create a new order that will require either growing and producing new cannabis or sourcing from another licensed producer.
Once the production order is approved (using the new Approval Workflow in AirMed), it will cascade out all related tasks to complete the order.
For instance, a producer makes a sale to a provincial distribution warehouse for 2000 vape cartridges. The producer can create a new batch of plants as source material and then track the entire process from propagation to finished packages in master-cases loaded on a pallet to be shipped.
The entire production process can be tracked and reported on, even if the workflows to produce the vape cartridges require a third-party to manage the extraction and packaging stages.
For more information call or email us, fill out the request demo contact form or visit our Software page.
Introducing Our Updated WordPress Medical Plugin
Elevate Your Medical Cannabis Website Experience
We’re excited to announce the release of our enhanced WordPress Medical Plugin — now better than ever to help you build a cutting-edge medical cannabis website in Canada.
The AirMed Medical Plugin seamlessly integrates with Wordpress websites, letting licensed Canadian cannabis producers launch a fully functional medical cannabis platform. No need to upload product information to a third-party ecommerce site. Your brands and products feed directly onto your website from your AirMed database — in real time.
Our latest update, driven by customer feedback, introduces powerful new features designed to make your website stand out in a competitive market.
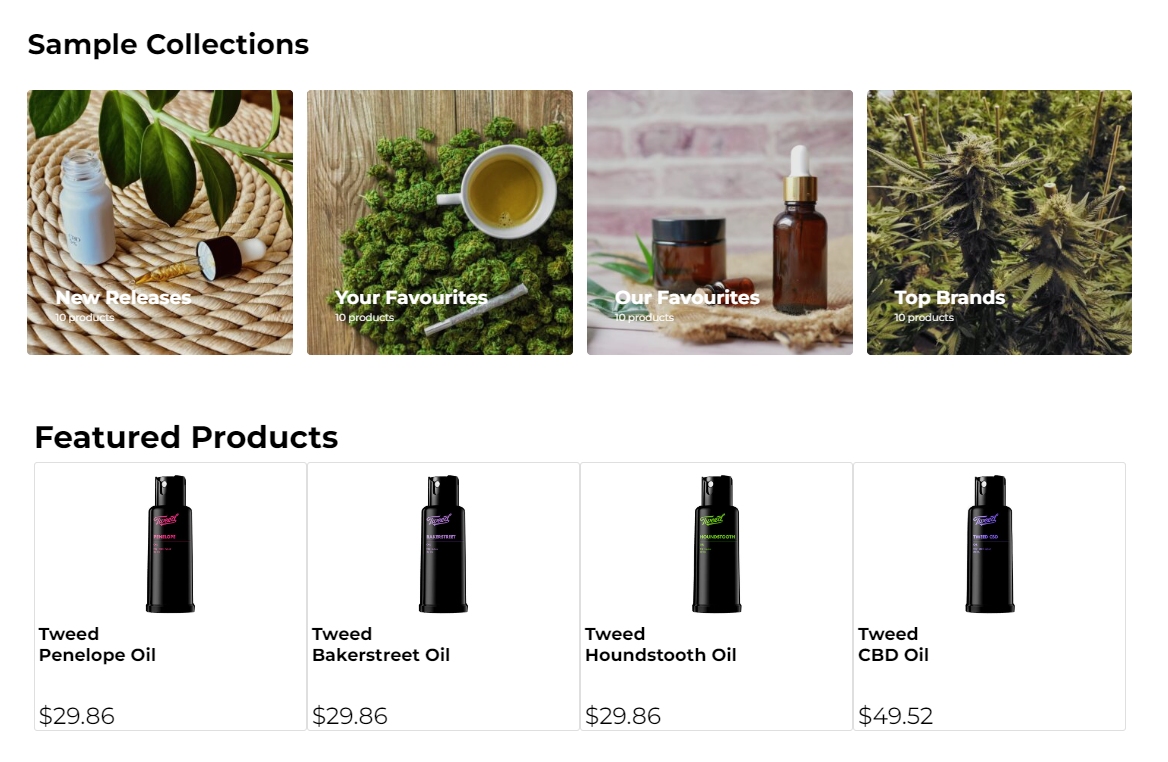
What’s New?
- Enhanced User Experience: We’ve added a sleek new carousel control, making it easier to showcase your products and services in a visually engaging way. Paired with optimized metatags, your site will not only look better but also attract more traffic through improved search engine visibility.
- Mobile-Friendly Design: Recognizing the importance of mobile users, we’ve updated all in-built webpages to deliver a seamless experience on smartphones and tablets. Your patients can now access crucial information, no matter where they are, with the same level of quality as on a desktop.
- Dynamic Dashboard Widgets: Stay on top of your site’s performance with our new dashboard widgets for summary pages. Get insights at a glance, making it easier than ever to manage and optimize your content.
With our plugin, patients can register, submit applications, and purchase medical cannabis — with all data securely encrypted in full compliance of Health Canada regulations.
As an AirMed customer, you can quickly install the plugin and add a simple WordPress shortcode to display your product catalog.
Whether you’re a clinic or medical cannabis producer, our updated WordPress Medical Plugin gives you the tools you need to create a compelling, user-friendly online presence.
Get started today and take your medical cannabis business to the next level!
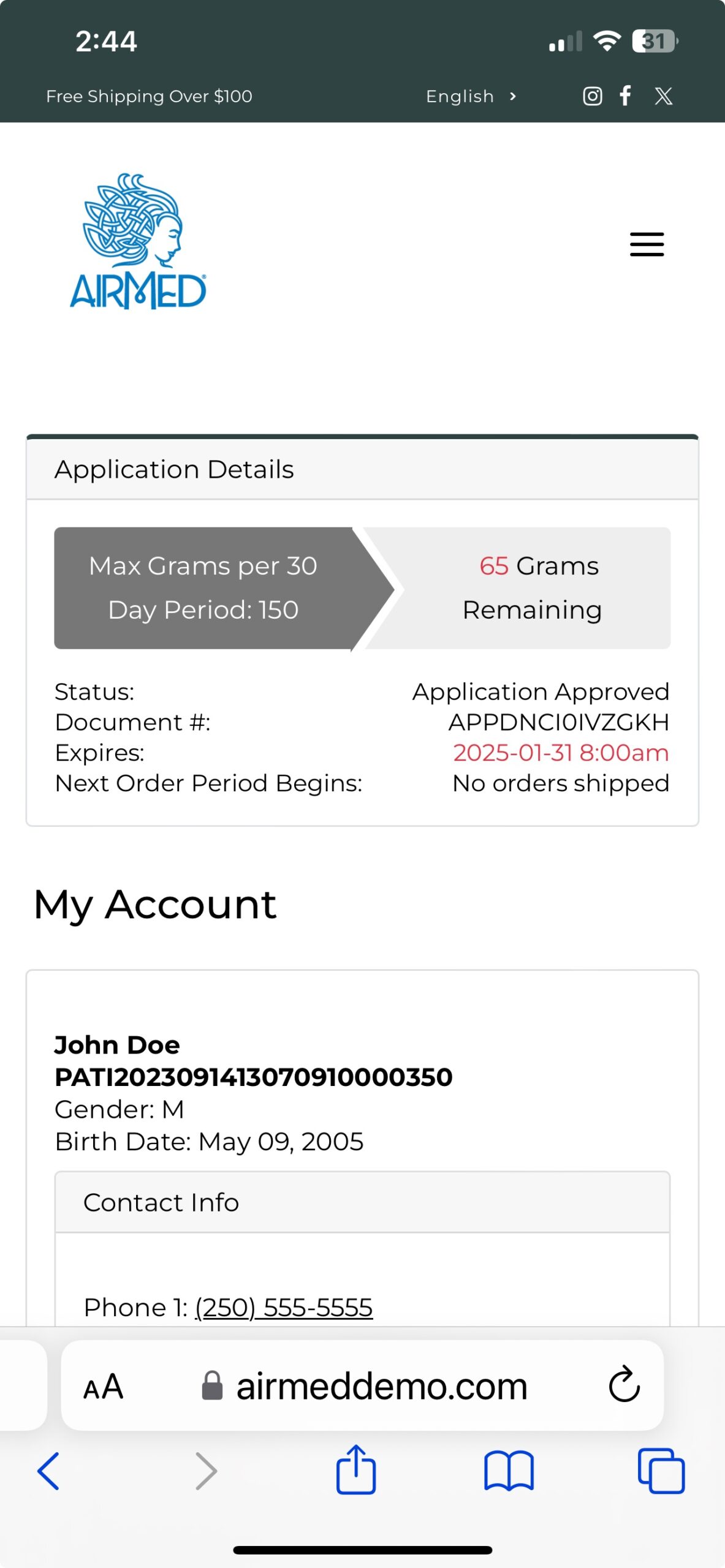
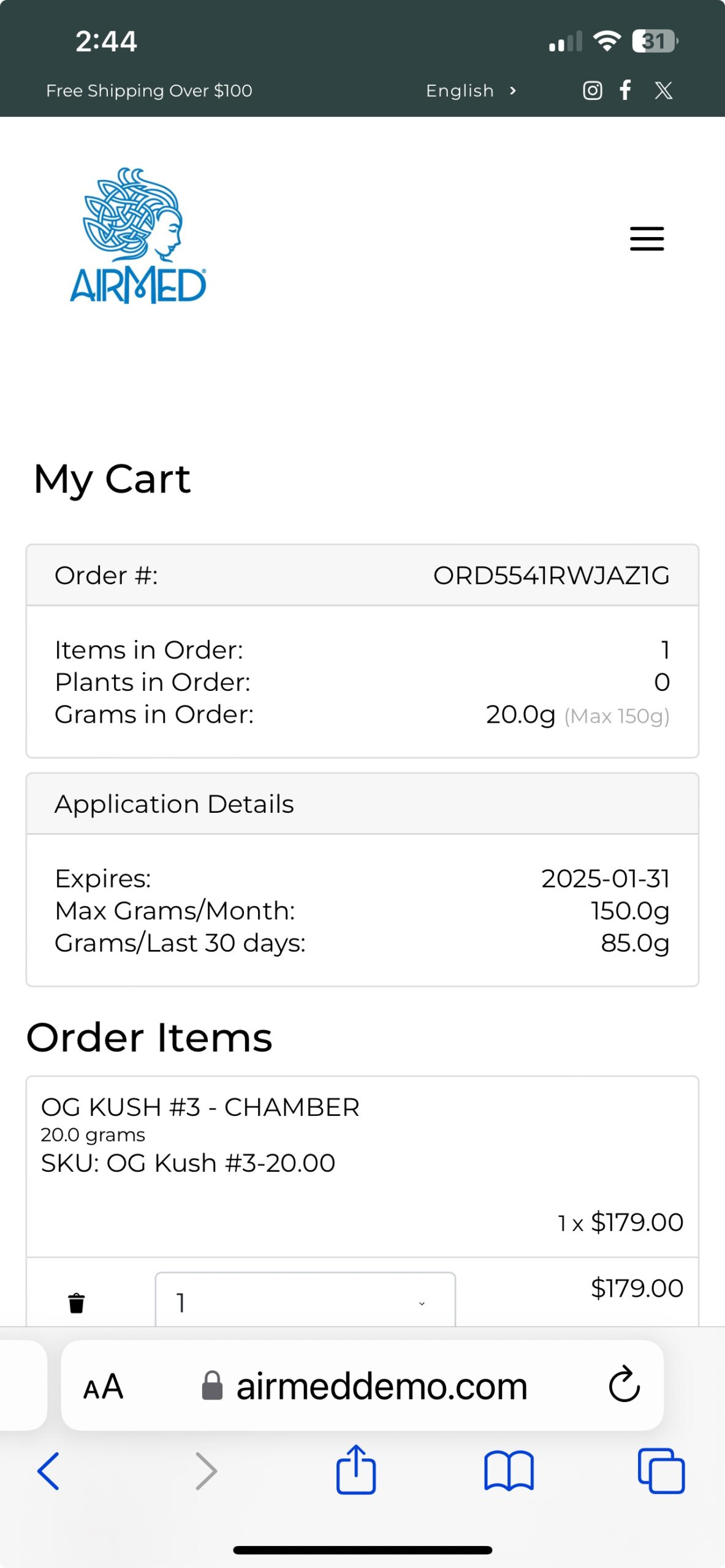
Call us toll-free at 1-877-313-2442 to learn how our plugin can help you achieve your goals in the medical cannabis marketplace.
To see our plugin in action, visit our live demo site at airmeddemo.com.
AirMed Supports Direct Delivery
What is Direct Delivery?
Direct delivery programs let licensed producers sell their products directly to retailers, which can eliminate warehousing. In British Columbia, the standard practice is for LPs to send cannabis to the BC Liquor Distribution Branch’s (LDB) warehouse. Dispensaries must then choose the products they wish to buy from the LDB product list.
For retailers, the disadvantages include limiting choices to what’s available in the warehouse and typically requiring a minimum order amount. This process also does not allow retailers to select products based on freshness.
For LPs, there is no assurance that the cannabis sent to the warehouse will be ordered within its shelf life. Yet each time a product is shipped, the production facility must use a pre-paid excise stamp. As a result, LPs spend the price of the excise stamp without a guarantee of sale. The possibility exists for the cost of producing and shipping the product to be thrown away, plus there’s the extra work involved in reclaiming the excise tax.
Direct delivery lets LPs register and list products for sale through a provincial direct-to-retailer program. Dispensaries can choose products from an individual LP and arrange for delivery directly from production facility to storefront. The shipment still requires an excise stamp and provincial fees, but the sale to the retailer has already been made, which covers those costs.
The benefits of direct delivery include:
- Giving retailers greater choice when ordering plus no minimum sales and potentially shorter delivery times
- Supplying dispensaries and consumers with fresher product
- Letting LPs cultivate-to-order and preventing products from expiring in warehouses
- Removing speculative costs of excise stamps for LPs and ensuring up-front revenue to LPs
- Helping small-scale producers be more competitive in the marketplace by creating opportunities for brand marketing and promoting better customer service to retailers
How AirMed Supports Direct Delivery
AirMed Direct Delivery offers a streamlined service that enables licensed retail stores to bypass provincial warehouses and order products directly from licensed producers who use AirMed in provinces where this is permitted, currently British Columbia and Saskatchewan.
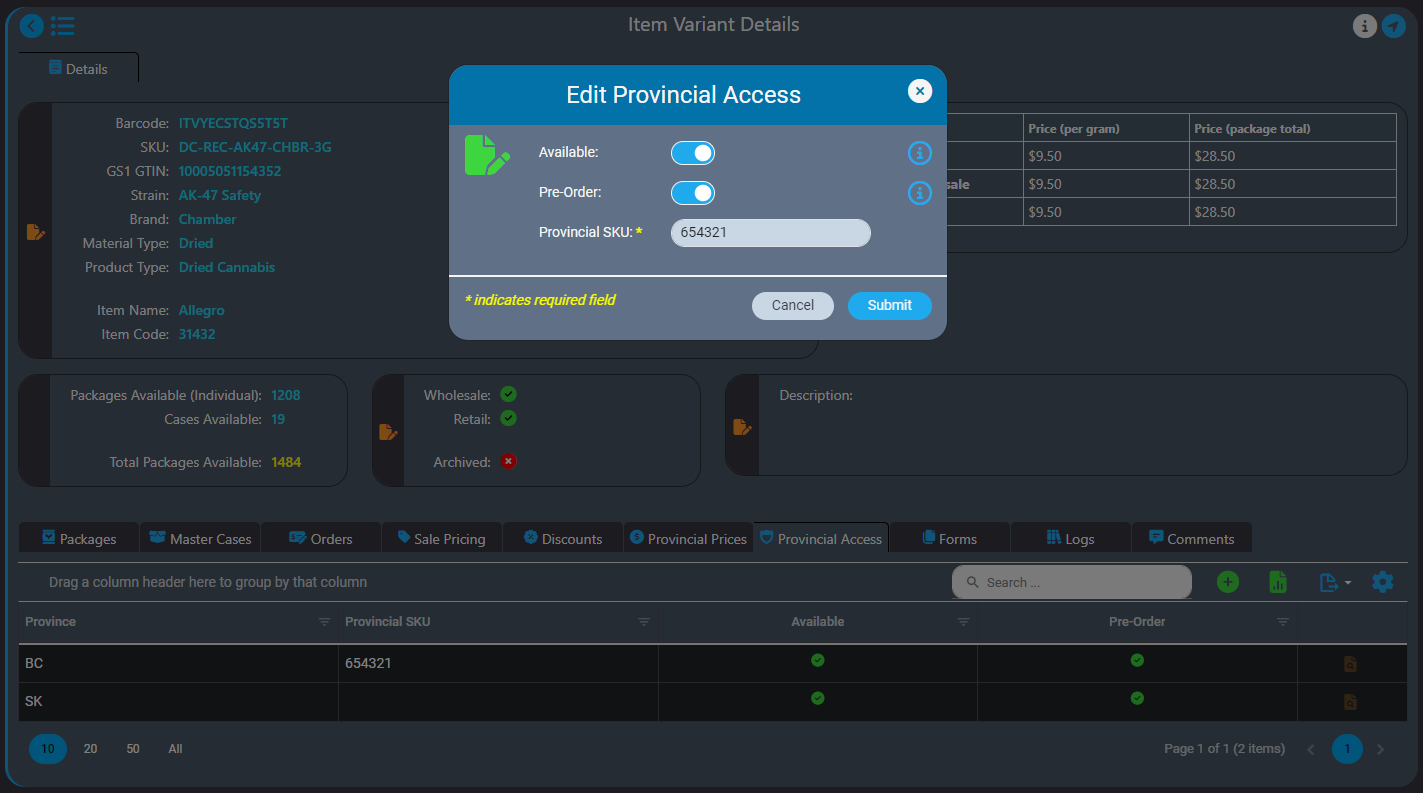
Our built-in workflows that support direct-delivery simplify your processes making it easier for you to work with your retail partners.
WordPress Plugin for Retailer Registration
A proprietary WordPress plugin and API provide fast and easy integration of your AirMed database with an online store on your website. This lets you offer your products directly to licensed retail outlets complete with a catalog and shopping cart. Retailers can register and get approval from licensed producers through the WordPress plugin. Approved retailers gain access to a product catalogue and order cases of finished packages.
Pricing and Orders
AirMed supports different pricing structures for various provinces for both individual packages and bulk cases. AirMed also allows pre-ordering of out-of-stock products, enabling producers to manufacture based on incoming orders.
Order Fulfillment
Once an order is placed, it is automatically processed in AirMed. Producers can fulfill orders, manage payments, and ship products directly to retail stores.
Provincial Support
Direct delivery is designed to comply with current provincial regulations and can adapt to include new provinces as regulations change. You can even set up direct-delivery product availability for provinces that may elect to allow direct delivery in the future.
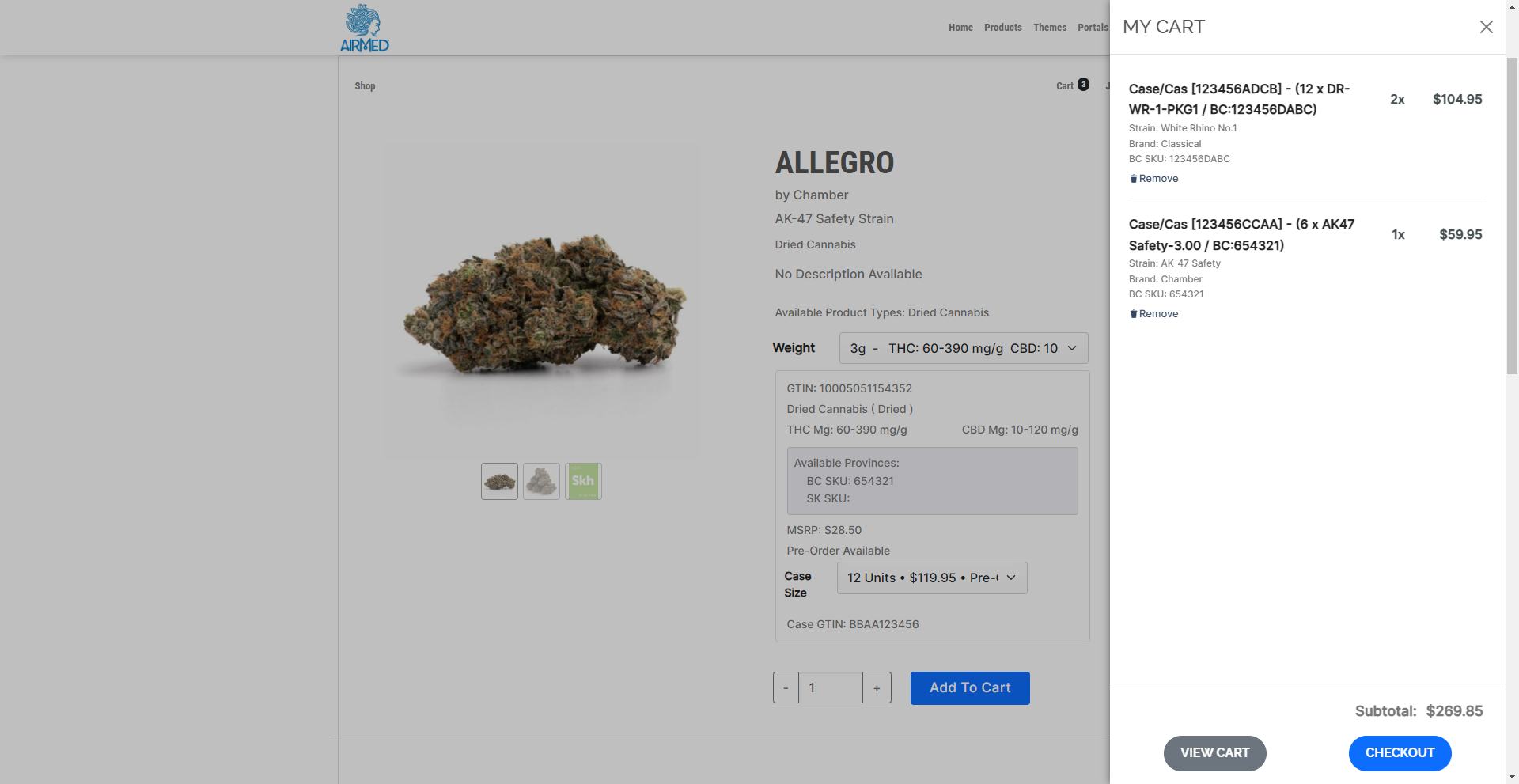
Our solution is ideal for those looking to streamline their supply chain and enhance their ordering process in the cannabis retail industry.
Efficiency: Retail stores can directly access products from producers who use AirMed, reducing the dependency on provincial warehouses and potentially shortening delivery times.
Flexibility: Producers can manage their inventory and production schedules more effectively through pre-orders, and AirMed manages creating product cases in varying sizes with associated case pricing for each product type and province.
Scalability: Our system can expand to include additional provinces as they permit direct delivery.
To see all the ways AirMed supports direct delivery, book a free demo by using the Request Demo button at the top of the page.
For more information on direct delivery in British Columbia, visit: https://www.bcldbcannabisupdates.com/LDBDirectDeliveryProgram
For more information on cannabis laws in Saskatchewan, visit: https://www.slga.com/cannabis
To read an article about direct delivery on the Stratcann website visit: https://stratcann.com/insight/bcs-
annabis-direct-delivery-program-is-growing-but-fees-still-too-high/c
Data collection made easy with AirMed e-form designer
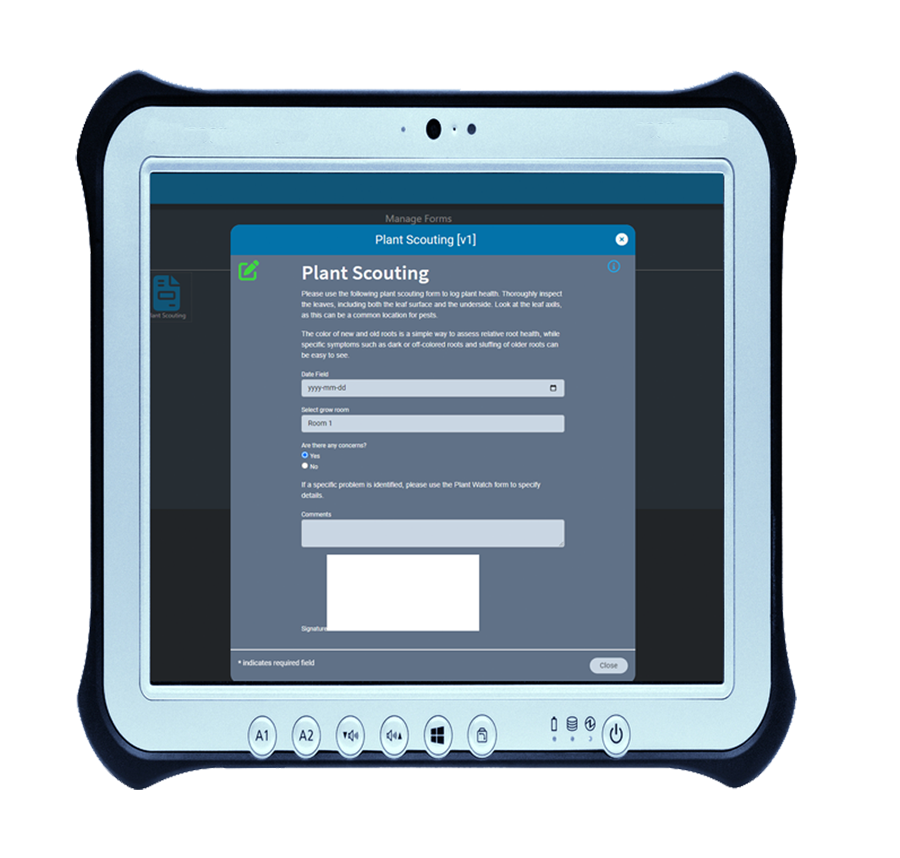
For many compliance-based businesses, forms are unavoidable. Even with the most comprehensive software, forms are sometimes necessary for collection and verification of data.
While many software vendors offer electronic form functionality, in most cases the information contained in those forms is not stored in the database. This means relying on PDFs or printed documents to access the data contained in the form.
Our built-in form designer lets you create custom electronic forms that integrate with AirMed’s system data-sources. All fields in electronic forms created using the form designer can be stored in the AirMed database. There is no need to refer to physical documents or PDFs.
Using AirMed, you can automate workflows using custom e-forms to prevent the need for paper-based documentation. Forms can be linked to processes such as plant scouting, nutrient checks, room sanitation, and shop floor data collection. Using our form designer, you can employ drop-down picklists, date & time, checkboxes, radio groups, custom fields and more. AirMed lets you apply auto-complete, file upload, hidden input and electronic signatures to your forms.
All the data is electronically accessible and even reportable.
Form data collection doesn’t get any easier than that.
For more information visit our AirMed 5 page.
Workforce Management in AirMed 5
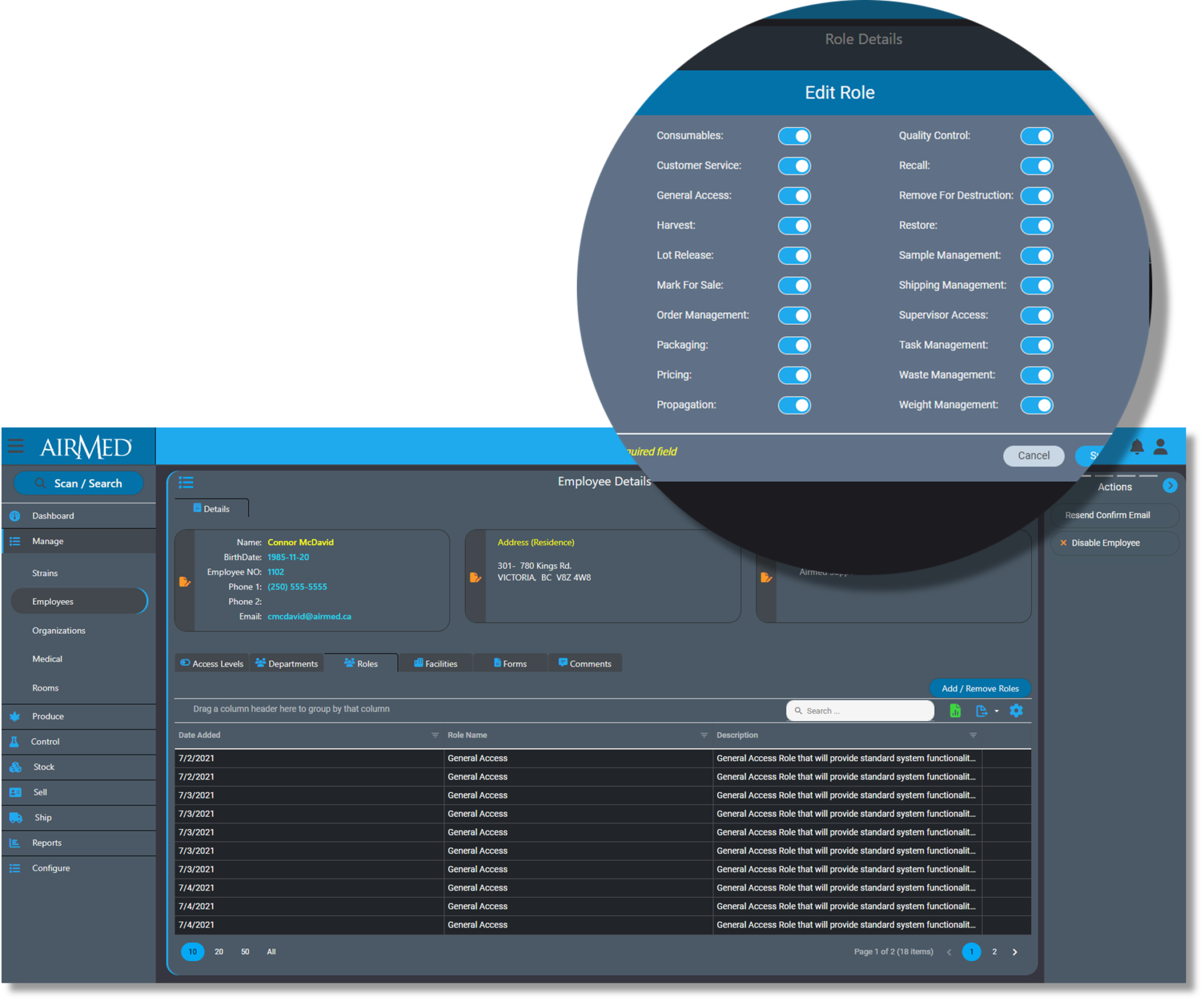
AirMed simplifies compliance by tracking and logging every action and ensures audit readiness with reports and analytics.
Employees only have access to functions and data that relates to their job responsibilities. System Administrators can only access data through approved computers utilizing a VPN connection.
And AirMed offers licensing by facility size rather than by individual user to encourage producers to configure individual accounts for every worker.
Our system improves quality management by allowing producers to identify gaps in employee knowledge and skills.
To see for yourself what AirMed can do, click the Request Demo button at the top of the page.
For more information visit our Software page.
Implementing AirMed in Your Facility

As a cloud-based platform, AirMed does not require expensive dedicated hardware to operate. Producers are free to use any computing device that supports a web browser. As a result, set up consists mainly of configuring the software to meet your needs. The rest of the time involves learning to use the system and getting your employees up and running.
How AirMed is implemented within your organization depends on the areas of the software you’ll be using. The first step in any software implementation is a needs assessment to help identify exactly how the software will be used. Understanding the capabilities of AirMed and how it can work with your processes is one of the keys to successful implementation. Your AirMed implementation specialist will work with you to perform an assessment of your needs to determine which areas of the software should be configured.
We provide an implementation guide and a production checklist that you can go through with your AirMed Implementation Specialist. Together, you’ll review the functionality in AirMed and determine which areas and functions are needed for your business operations. AirMed includes the ability to disable navigation menus for areas that you won’t be utilizing to provide a streamlined interface for your workers.
When you have completed your assessment and training, the next step is to configure your Live Production environment. After you’ve set up and tested your system, it’s time to train other users (your workers) to use AirMed.
Once users have usernames and passwords, they can access the training resources in the AirMed Learn environment. Workers can take additional training at a later date to develop skills for different areas of the system — in fact, anyone with access to AirMed can use the Learn environment at any time to practice new workflows or refresh their knowledge. When employees have completed training, you can give them access to the system, so they can get to work.
With AirMed you can implement only the functionality you need right now. For example, you can start by implementing the AirMed Grow module, then when you expand your operation, you add modules that meet your current needs for performing extractions, packaging for provincial sales, selling to medical patients or whatever your business entails.
Working with your implementation specialist will help you see all the benefits of using AirMed and ensure that you are getting the most from our software.

For more information on how AirMed helps specific types of businesses, visit our Customers page or our Frequently Asked Questions page.
Ready to learn more about AirMed? Click the Request Demo button or call 1-877-313-2442.
AirMed and FDA CFR 21

We are sometimes asked if AirMed meets FDA standards. First, please be aware that the FDA is a department of the US government. The specific portion of FDA regulations relevant to software such as AirMed is part CFR 21. As it is an American standard, Health Canada does not require CFR Part 21 compliance as part of the Cannabis Act Regulations. And while AirMed was designed for the Canadian cannabis industry and to comply with Health Canada regulations, AirMed does conform to CFR Part 21 with respect to electronic record keeping, audit trails, and electronic signatures.
Many agencies throughout the world are responsible for issuing and enforcing regulations that affect businesses. The regulations affecting software such as AirMed are typically those related to records management compliance. And while there are many regulatory agencies involved in records management, for the most part the regulations themselves are similar from country to country and agency to agency. The purpose of them, in general, is to ensure the security, confidentiality and authentication of electronic records.
The U.S. FDA regulates food, drugs, medical devices, biologics, animal feed and drugs, cosmetics and radiation-emitting products such as cell phones for the U.S.A. The FDA’s rules for manufacturing and distribution are designed to protect consumers and promote public health. In the U.S. Code of Federal Regulations (CFR), Title 21 deals with Food & Drugs. Until recently, the regulations in this title required paper records with handwritten signatures.
Back in 1997, part 11 of 21 CFR was enacted to cover the use of electronic records and electronic signatures. Commonly known as 21 CFR 11, this part defines the criteria “under which the agency considers electronic records, electronic signatures, and handwritten signatures executed to electronic records to be trustworthy, reliable, and generally equivalent to paper records and handwritten signatures executed on paper.”
Essentially, the concerns about using electronic records are that records may be lost in a system crash, the data may become corrupt or modifications may be made without proper authorization. In addition, since printed documents with hand-written signatures are recognized as legally binding on the signators, the agencies are looking for ways to make electronic records similarly binding on their owners. The regulations have been proposed to ensure that whenever an organization replaces printed documents with electronic data, there are checks and balances in place to ensure integrity of the electronic records so that they can be legally equivalent to printed records.
AirMed has a range of features that satisfy standards for security, authentication, validation and auditing as outlined in 21 CFR 11 and other regulations.
For more detailed information visit: Code of Federal Regulations (CFR) | FDA
For more information on how AirMed helps you meet compliance, visit our Compliance page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
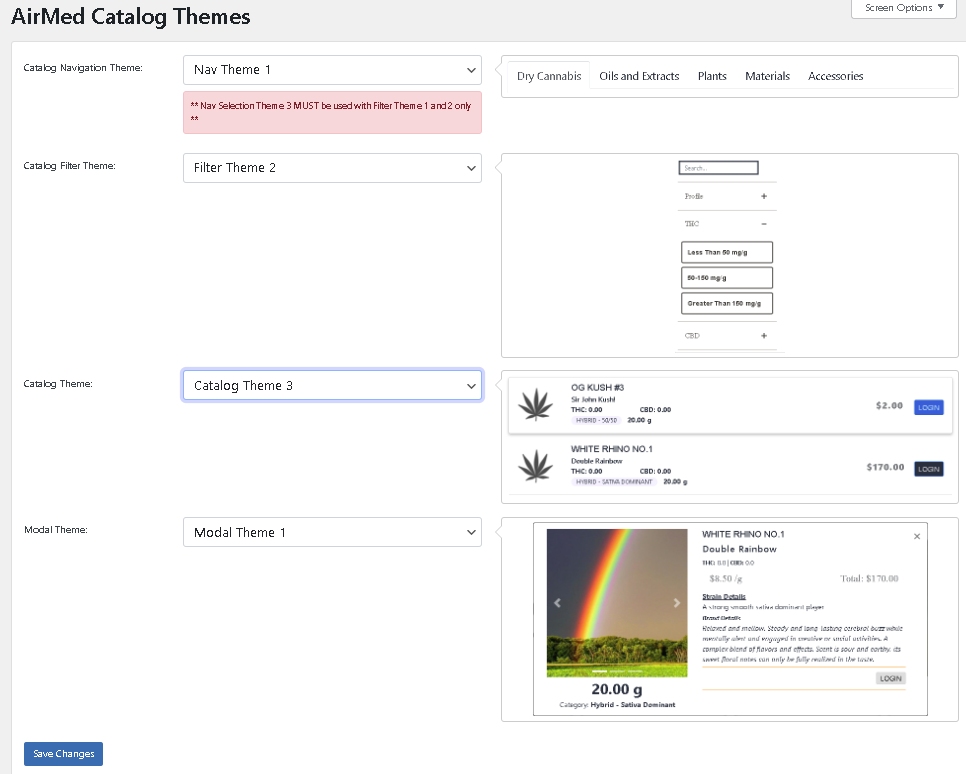
AirMed Plugin for Your WordPress Website
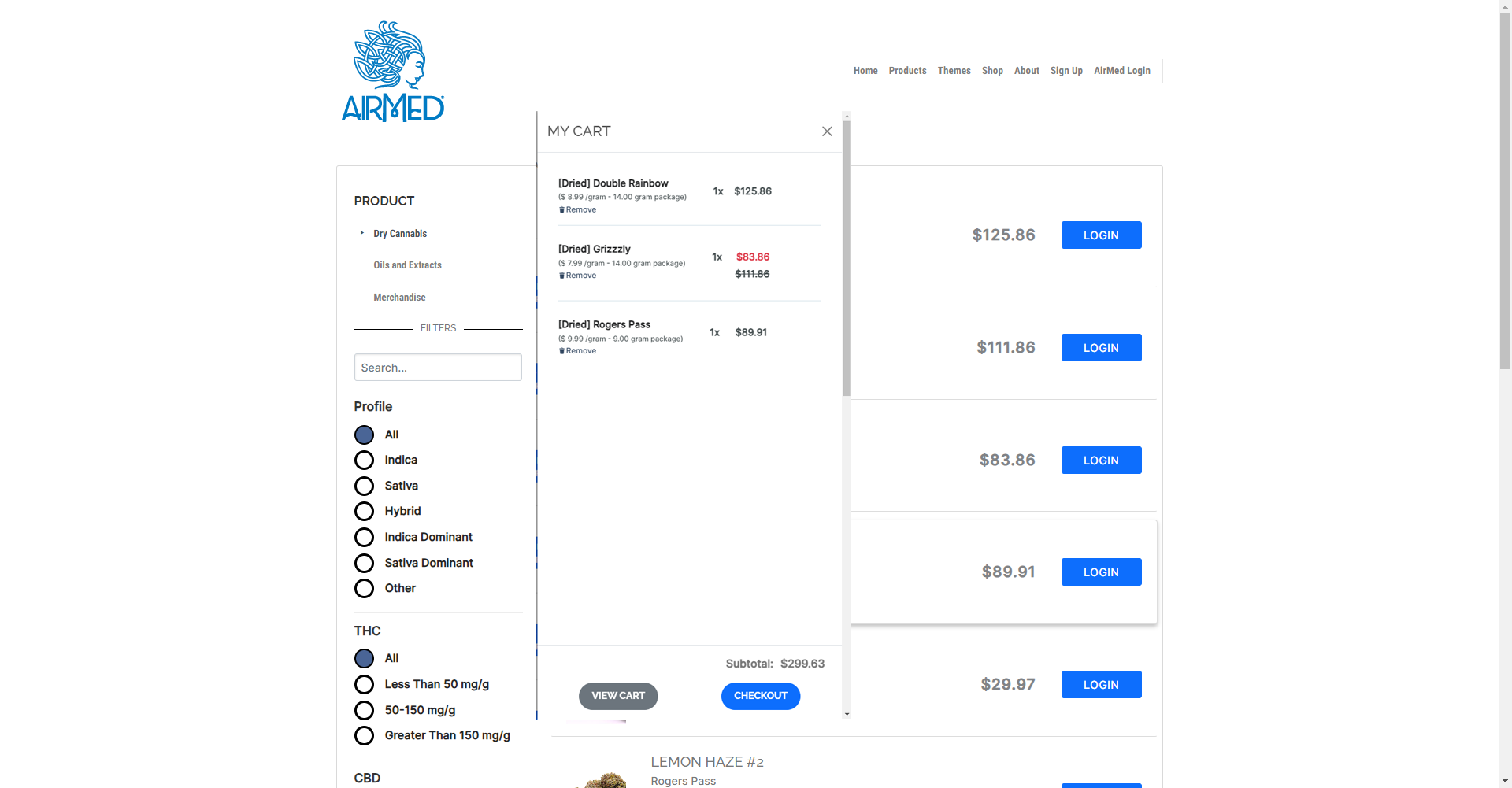
The AirMed WordPress plugin can pull information from your AirMed account to showcase your brands and products in an online catalog. Content stored in your AirMed database is published directly to your WordPress web pages.
Use the pre-designed theme options in your AirMed plugin settings to determine how your catalog will appear on your website.
View your choices in a visual interface installed on your WordPress dashboard. Save the settings to see the finished result on your web page.
Although the catalog appears on your website, all product details are stored in AirMed. Payments are processed by the merchant solution provider with no card data passing through AirMed. Patient information is encrypted by Canada’s most secure hosting facility. With support for monitored intrusion prevention and monitored firewall, your records have the highest level of protection.
As an AirMed customer, you install a simple plugin onto your WordPress website. The plugin installs a visual interface in your WordPress dashboard.
Then add a snippet of shortcode to the page where you want your catalog to appear. The shortcode pulls directly from your AirMed database, which contains all your products complete with images, descriptions and pricing.
Customers interacting with the product catalog remain on your website throughout the shopping experience rather than being transferred to a portal site.
The WordPress plugin interfaces with our application programming interface (API) to embed information from the AirMed database directly onto your website.
Change the style, format, and position of catalog elements using the plugin settings and see how the elements will appear on your website before committing. When you find the combination you like, save your settings and refresh the web page.
For more information on these new features or to book a demo of AirMed to see them for yourself, click the Request Demo button at the top of the page or use any of the contact forms.
In the meantime visit our Intro to AirMed 5 page.
Intro to AirMed5 Booklet

We’re so excited about the release of AirMed5 that we’ve created a booklet describing the new software in detail. This document covers all the features in the initial release.
- Re-imagined interface and user experience
- Fully customizable tables with custom columns
- Built-in report, label and e-form designers
- Business intelligence designer
- SOP library with built-in SOP editor
- Automated QMS incident management
- Task-based workflows with support for task templates and automated task scheduling
- Product plans
- Simplified packaging and shipping
To receive a PDF of this booklet by email, please send a request to sales @ airmed.ca or fill out one of our request demo contact forms.
In the meantime, visit our AirMed 5 web page.
Redesigned Order Screen in AirMed5

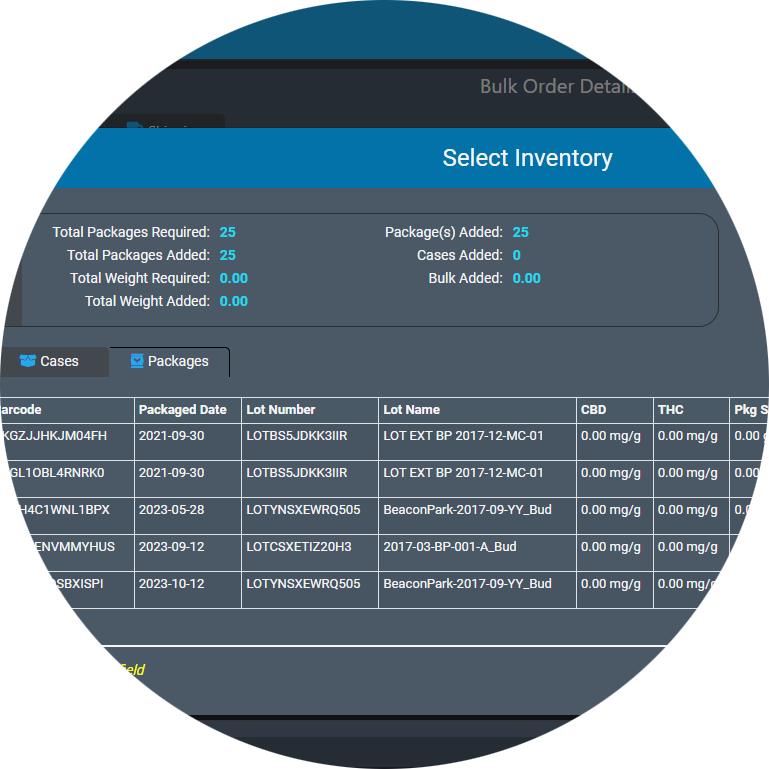
AirMed5 lets you manage order requests, order planning, order assembly, and shipping from one screen.
After releasing a planned order to production, verify order items in one step. Once you have entered the customer requested products and quantities, browse available inventory from multiple lots to plan the order fulfillment.
The new order processing in AirMed5 offers unparalleled ease while managing complex order requirements.
Manage products and different package types using the new Items table. Items and Item variants let you easily browse and manage products, different package sizes and available inventory.
Create new package runs and pack cases with streamlined workflows. Manage bulk items, recreational products, and medical products.
For more information on these new features or to book a demo of AirMed to see them for yourself, click the Request Demo button at the top of the page or use any of the contact forms.
In the meantime visit our Software page.
We’re streamlining so you can work faster
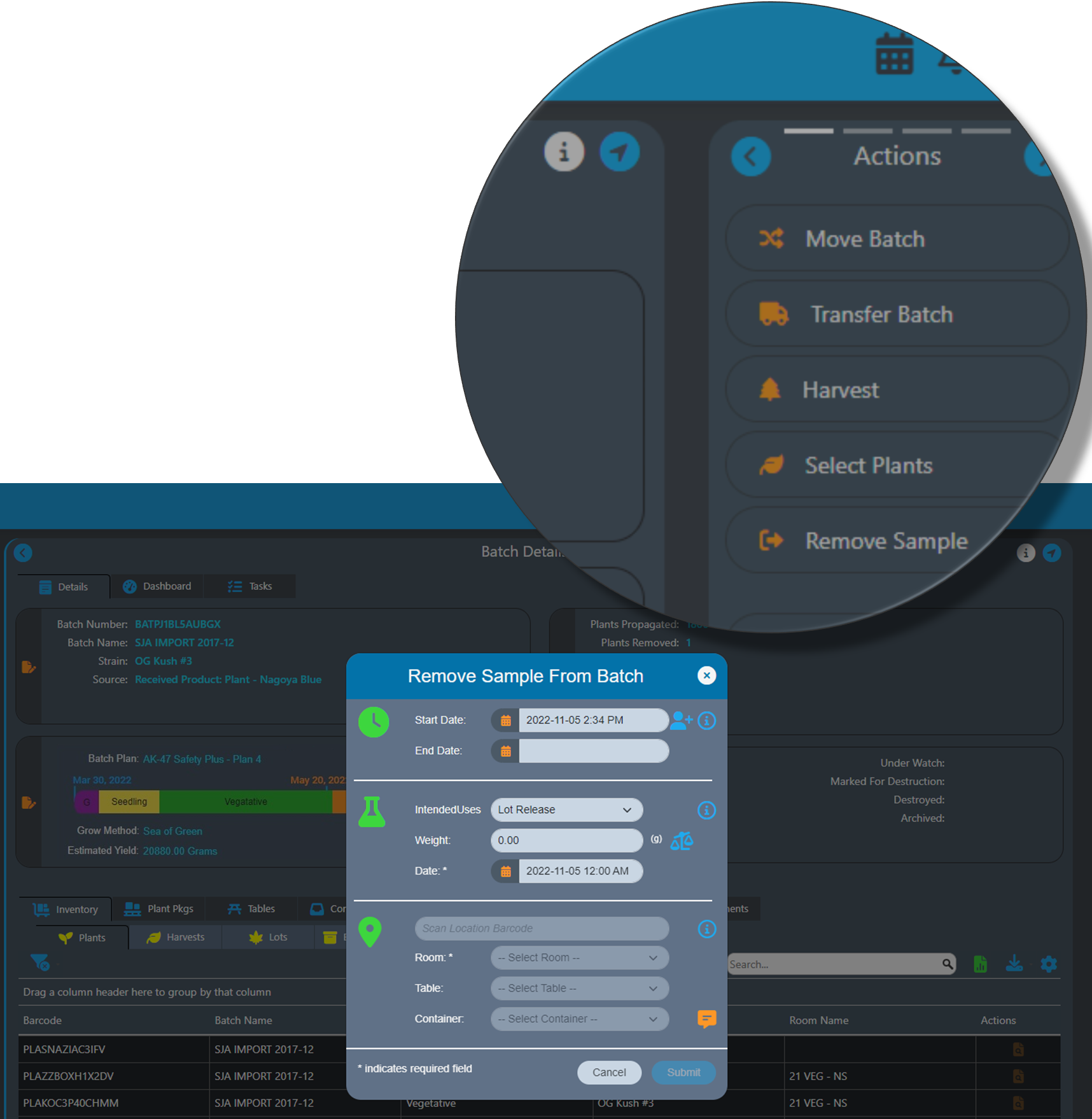
AirMed 5 streamlines processes so that you can work faster and more efficiently. Each action workflow has been redesigned to minimize the number of clicks required to complete the step.
Comments are available in a pop up, and lists can be auto populated by scanning a barcode. To select a location, it’s possible to scan the barcode for a room, table, or container, and all the pick-list values will be selected.
Smart input fields ensure erroneous data cannot be entered. If the database contains only a certain number of items, the system will not allow a higher number to be entered. Date fields are automatically restricted based on other data in the system so that it becomes impossible to back-date an item to a date before the item was recorded in the system.
Required fields in action dialogs are available on the first screen the user encounters.
And AirMed 5 extends the actions menu with a carousel to show Actions, Reports, SOPs, and Forms. The Report, SOP, and Form sections have customizable lists where users can select the items that are relevant to them.
For more information on these new features or to book a demo of AirMed to see them for yourself, click the Request Demo button at the top of the page or use any of the contact forms.
In the meantime visit our Software page.