AirMed 5 Introduces Inventory Ledgers
AirMed 5 now features powerful new functionality to simplify inventory management and improve recordkeeping. With the integration of inventory ledgers throughout the system, you can effortlessly track all transactions related to your inventory.
Comprehensive Tracking
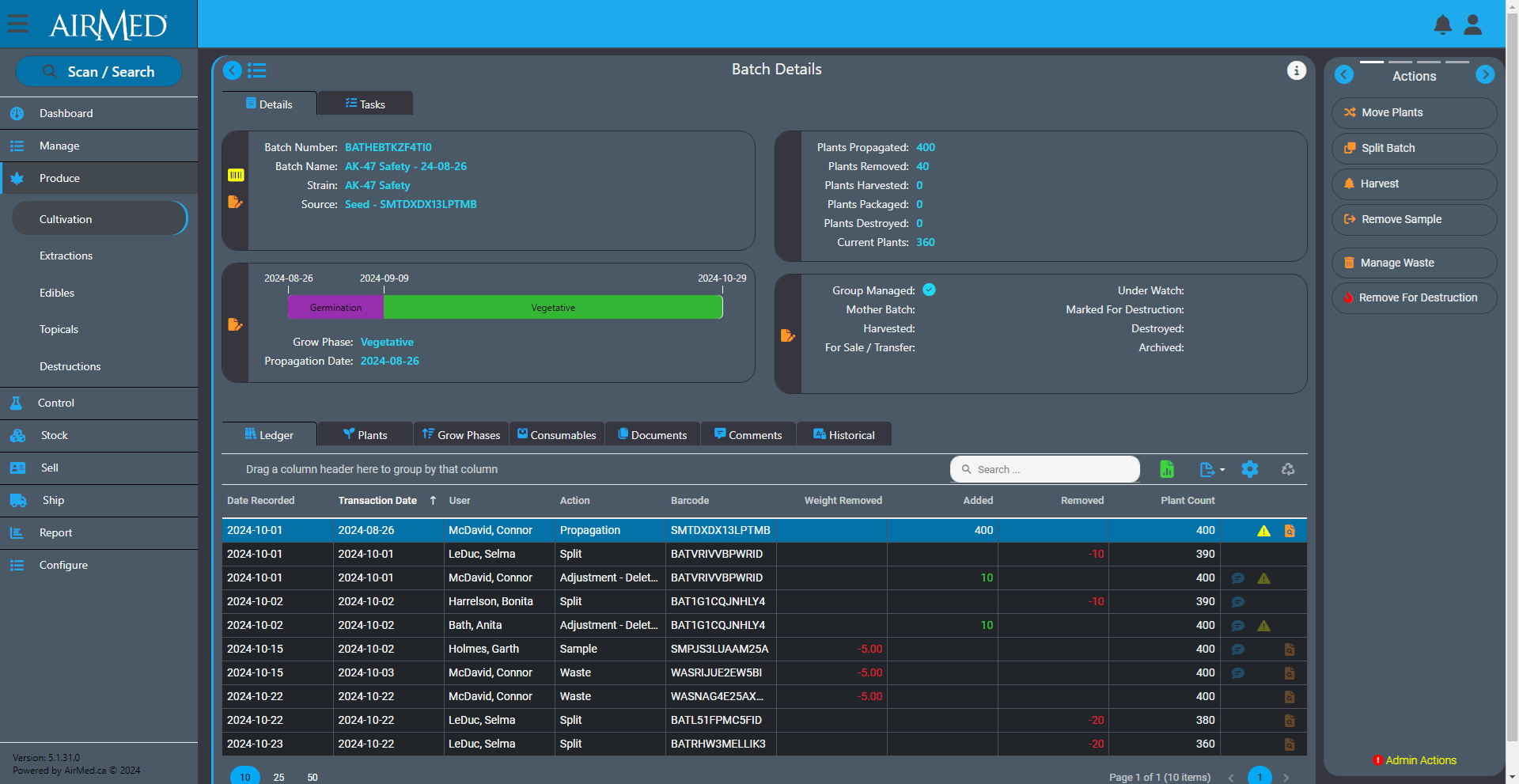
Inventory ledgers provide detailed tracking of every addition or reduction to inventory, whether in batches or lots. Each entry includes the user responsible for the transaction, along with the date, time, transaction type, and the corresponding weight or quantity. This ensures full visibility into the usage of each batch and lot, providing a clear audit trail. Additionally, clickable links enable quick access to related records and associated comments.
Intelligent Backdating
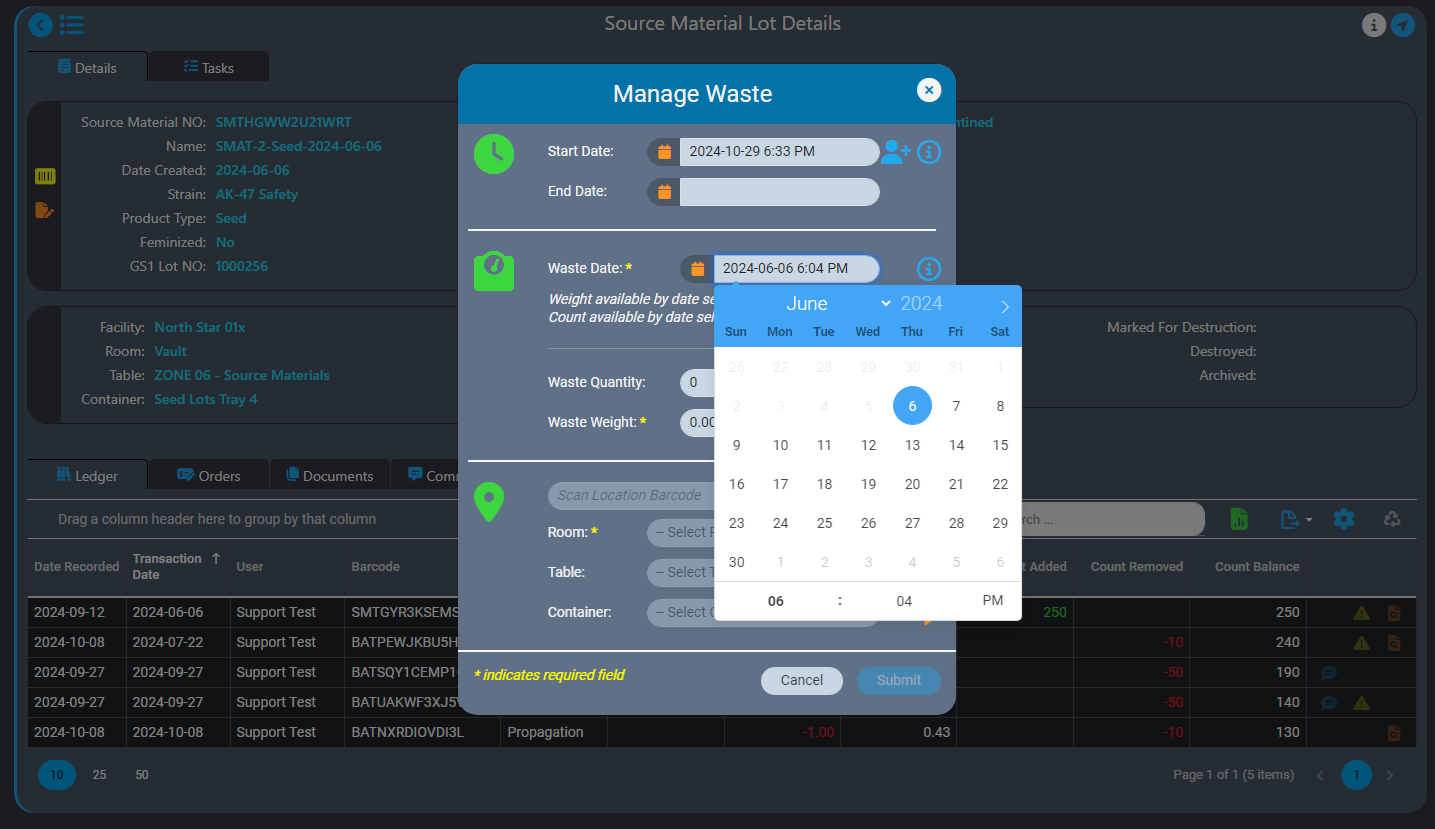
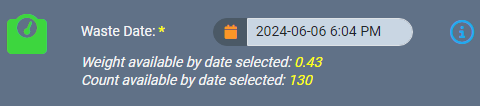
Intelligent record backdating ensures that adjustments or entries made in the past do not cause discrepancies in future records or reporting. For example, if waste for a source material needs to be logged, AirMed ensures that the waste entry can be backdated only after the source material was created. The ledger also prevents negative balances by automatically validating that backdated weights or quantities are consistent with subsequent records. If a backdated action impacts monthly compliance reporting, AirMed will automatically generate an incident within our built-in Quality Management System (QMS).
Efficient Adjustments & Corrections
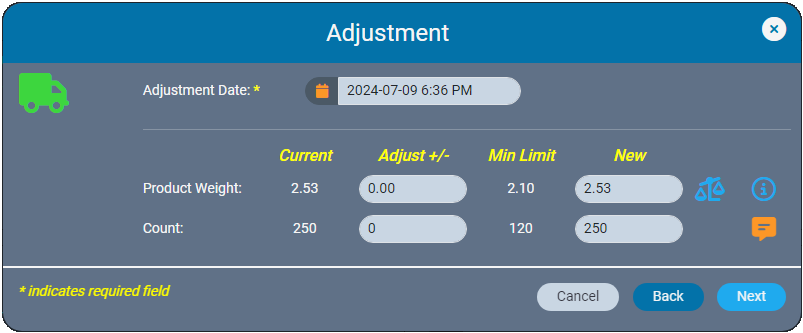
Inventory ledgers make it easy to adjust weights and counts to resolve discrepancies. AirMed ensures that backdated adjustments are logically aligned with all prior and subsequent records. Adjustments that affect inventory levels are clearly categorized as ‘other additions’ or ‘other reductions’ in monthly compliance reports.
Error Correction Options

In the event of data entry errors, AirMed offers multiple correction tools. For example, if an inventory item is created with the wrong date, admin tools can be used to correct the creation date to match the creation of the parent record (e.g., adjusting a lot’s creation date to align with the date of the harvest). All changes are logged in both the affected item’s ledger and the parent record’s ledger. Additionally, new options are available to delete incorrect records or merge materials back into their original source when appropriate.
The benefits of our new inventory ledgers include more accurate tracking, improved operational efficiency and easier compliance. For more information about AirMed 5 visit our Software page.
Introducing Our Updated WordPress Medical Plugin
Elevate Your Medical Cannabis Website Experience
We’re excited to announce the release of our enhanced WordPress Medical Plugin — now better than ever to help you build a cutting-edge medical cannabis website in Canada.
The AirMed Medical Plugin seamlessly integrates with Wordpress websites, letting licensed Canadian cannabis producers launch a fully functional medical cannabis platform. No need to upload product information to a third-party ecommerce site. Your brands and products feed directly onto your website from your AirMed database — in real time.
Our latest update, driven by customer feedback, introduces powerful new features designed to make your website stand out in a competitive market.
What’s New?
- Enhanced User Experience: We’ve added a sleek new carousel control, making it easier to showcase your products and services in a visually engaging way. Paired with optimized metatags, your site will not only look better but also attract more traffic through improved search engine visibility.
- Mobile-Friendly Design: Recognizing the importance of mobile users, we’ve updated all in-built webpages to deliver a seamless experience on smartphones and tablets. Your patients can now access crucial information, no matter where they are, with the same level of quality as on a desktop.
- Dynamic Dashboard Widgets: Stay on top of your site’s performance with our new dashboard widgets for summary pages. Get insights at a glance, making it easier than ever to manage and optimize your content.
With our plugin, patients can register, submit applications, and purchase medical cannabis — with all data securely encrypted in full compliance of Health Canada regulations.
As an AirMed customer, you can quickly install the plugin and add a simple WordPress shortcode to display your product catalog.
Whether you’re a clinic or medical cannabis producer, our updated WordPress Medical Plugin gives you the tools you need to create a compelling, user-friendly online presence.
Get started today and take your medical cannabis business to the next level!
Call us toll-free at 1-877-313-2442 to learn how our plugin can help you achieve your goals in the medical cannabis marketplace.
To see our plugin in action, visit our live demo site at airmeddemo.com.
AirMed Supports Direct Delivery
What is Direct Delivery?
Direct delivery programs let licensed producers sell their products directly to retailers, which can eliminate warehousing. In British Columbia, the standard practice is for LPs to send cannabis to the BC Liquor Distribution Branch’s (LDB) warehouse. Dispensaries must then choose the products they wish to buy from the LDB product list.
For retailers, the disadvantages include limiting choices to what’s available in the warehouse and typically requiring a minimum order amount. This process also does not allow retailers to select products based on freshness.
For LPs, there is no assurance that the cannabis sent to the warehouse will be ordered within its shelf life. Yet each time a product is shipped, the production facility must use a pre-paid excise stamp. As a result, LPs spend the price of the excise stamp without a guarantee of sale. The possibility exists for the cost of producing and shipping the product to be thrown away, plus there’s the extra work involved in reclaiming the excise tax.
Direct delivery lets LPs register and list products for sale through a provincial direct-to-retailer program. Dispensaries can choose products from an individual LP and arrange for delivery directly from production facility to storefront. The shipment still requires an excise stamp and provincial fees, but the sale to the retailer has already been made, which covers those costs.
The benefits of direct delivery include:
- Giving retailers greater choice when ordering plus no minimum sales and potentially shorter delivery times
- Supplying dispensaries and consumers with fresher product
- Letting LPs cultivate-to-order and preventing products from expiring in warehouses
- Removing speculative costs of excise stamps for LPs and ensuring up-front revenue to LPs
- Helping small-scale producers be more competitive in the marketplace by creating opportunities for brand marketing and promoting better customer service to retailers
How AirMed Supports Direct Delivery
AirMed Direct Delivery offers a streamlined service that enables licensed retail stores to bypass provincial warehouses and order products directly from licensed producers who use AirMed in provinces where this is permitted, currently British Columbia and Saskatchewan.
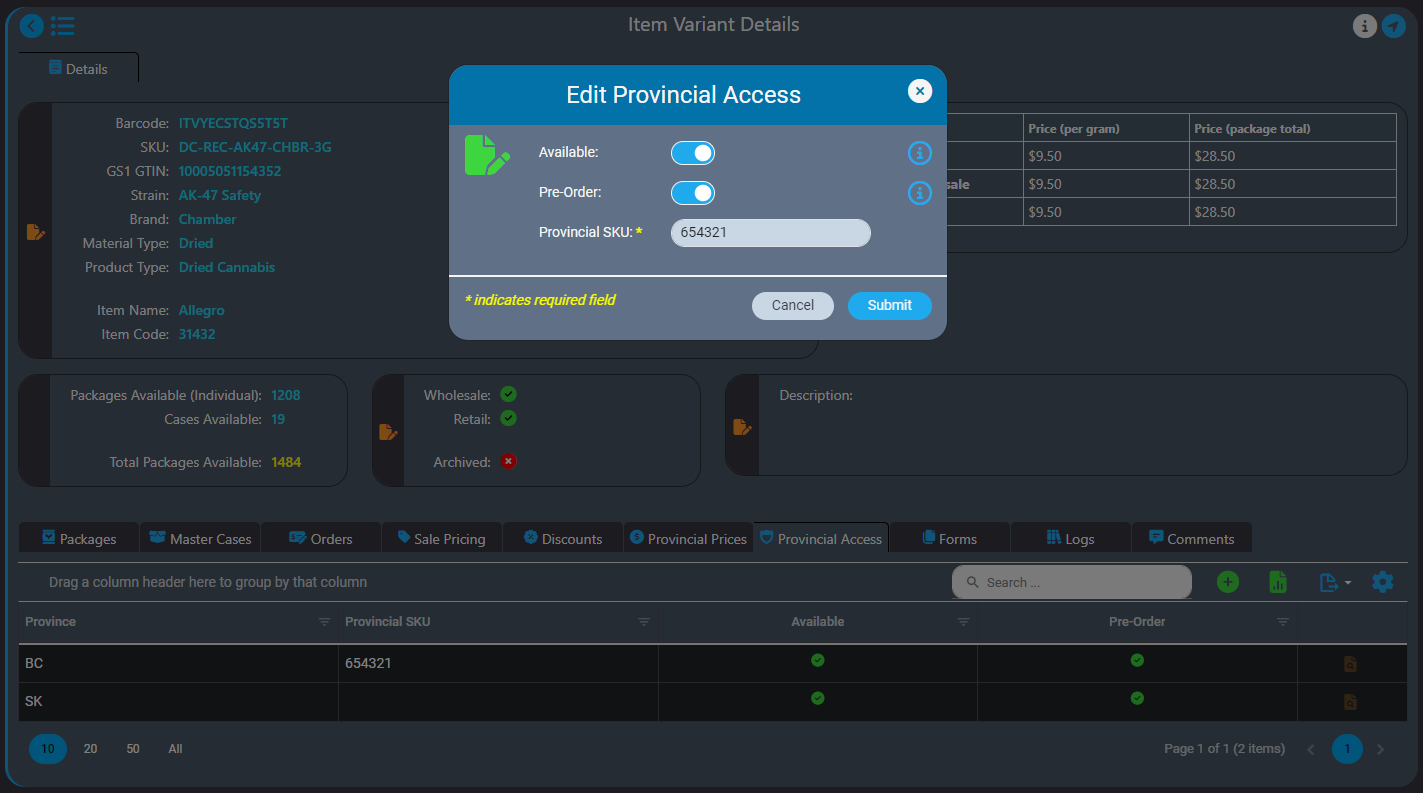
Our built-in workflows that support direct-delivery simplify your processes making it easier for you to work with your retail partners.
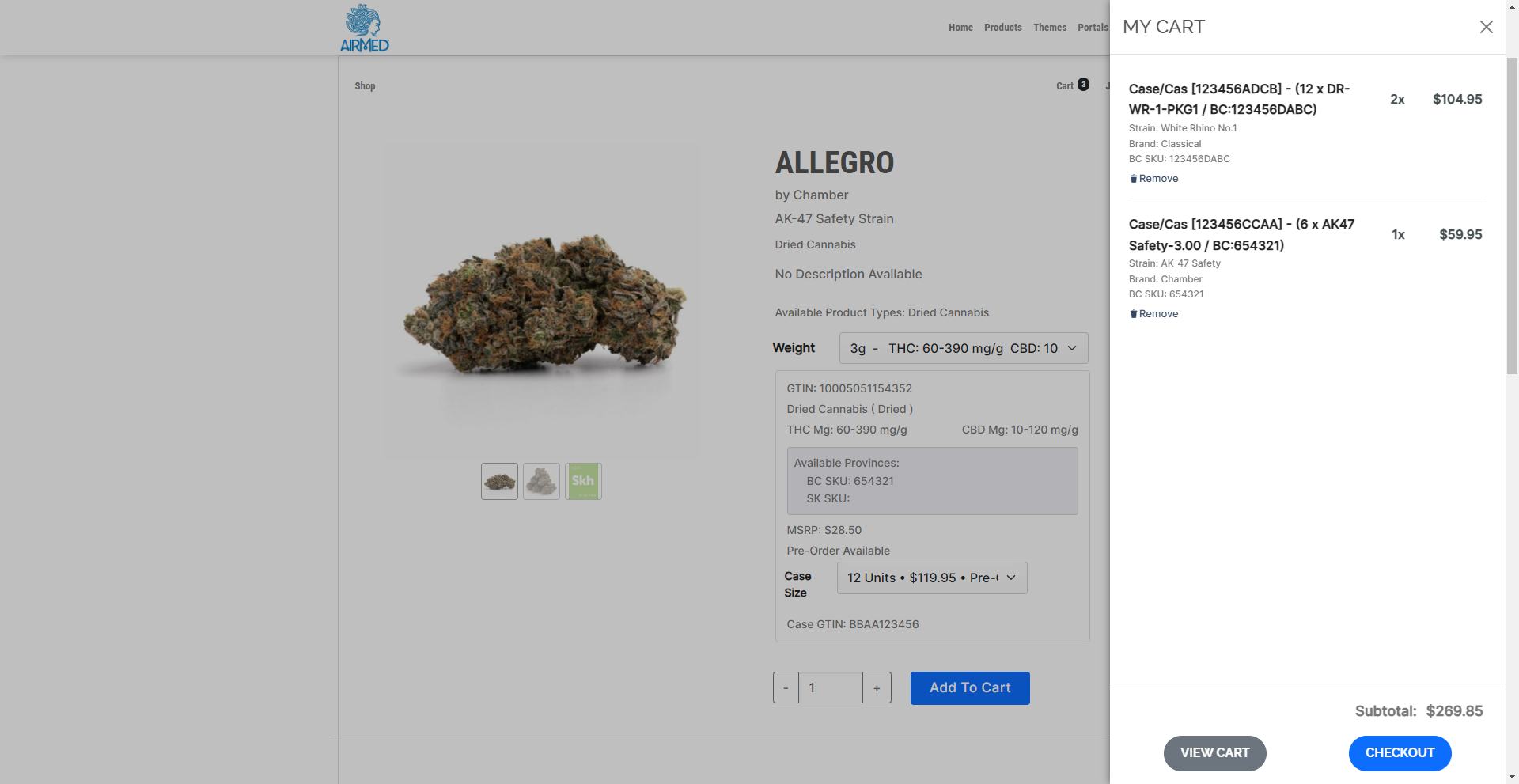
WordPress Plugin for Retailer Registration
A proprietary WordPress plugin and API provide fast and easy integration of your AirMed database with an online store on your website. This lets you offer your products directly to licensed retail outlets complete with a catalog and shopping cart. Retailers can register and get approval from licensed producers through the WordPress plugin. Approved retailers gain access to a product catalogue and order cases of finished packages.
Pricing and Orders
AirMed supports different pricing structures for various provinces for both individual packages and bulk cases. AirMed also allows pre-ordering of out-of-stock products, enabling producers to manufacture based on incoming orders.
Order Fulfillment
Once an order is placed, it is automatically processed in AirMed. Producers can fulfill orders, manage payments, and ship products directly to retail stores.
Provincial Support
Direct delivery is designed to comply with current provincial regulations and can adapt to include new provinces as regulations change. You can even set up direct-delivery product availability for provinces that may elect to allow direct delivery in the future.
Our solution is ideal for those looking to streamline their supply chain and enhance their ordering process in the cannabis retail industry.
Efficiency: Retail stores can directly access products from producers who use AirMed, reducing the dependency on provincial warehouses and potentially shortening delivery times.
Flexibility: Producers can manage their inventory and production schedules more effectively through pre-orders, and AirMed manages creating product cases in varying sizes with associated case pricing for each product type and province.
Scalability: Our system can expand to include additional provinces as they permit direct delivery.
To see all the ways AirMed supports direct delivery, book a free demo by using the Request Demo button at the top of the page.
For more information on direct delivery in British Columbia, visit: https://www.bcldbcannabisupdates.com/LDBDirectDeliveryProgram
For more information on cannabis laws in Saskatchewan, visit: https://www.slga.com/cannabis
To read an article about direct delivery on the Stratcann website visit: https://stratcann.com/insight/bcs-
annabis-direct-delivery-program-is-growing-but-fees-still-too-high/c
Selling internationally? You need GS1 and GTIN!

GTIN or Global Trade Item Number is a standard from the GS1 (Global Standards) organization. GTIN consists of unique codes that identify manufacturers and their products using barcodes. When scanned by an electronic reader, the GTIN barcode provides a code that is related to a specific manufacturer and a specific product from that manufacturer.
In North American, the UPC (Universal Product Code) is an existing form of the GTIN. In Europe, EAN-13 is the GTIN standard.
The GTIN system lets you and your products be identified across the globe. If you hope to sell in certain parts of the world, such as Europe, you will need to use GS1 standards and GTIN codes. Once you have signed up with your regional GS1 office, you will be issued a series of unique codes to use on your product packaging.
Fully supporting GS1, AirMed prints your GTIN barcodes directly from the database. Our master case processing lets you use multi-level barcoding for several layers of packaging or stock-keeping units (SKU). For example, one case could contain a dozen smaller cartons. Each of those cartons could contain a dozen retail-ready individual packages. All of those packaging layers can have its own barcode to meet the retail standards of the region where it will eventually be sold.
AirMed helps you meet your barcoding and packaging standards whether selling to a provincial warehouse or shipping internationally. AirMed even lets you apply pricing at the package or the master case SKU, and custom pricing can be set for specific SKUs by customer.
For more information on GS1 standards and GTIN barcoding, visit: https://gs1ca.org/
If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or click the Request Demo button at the top of the page to start the ball rolling.
Data collection made easy with AirMed e-form designer
For many compliance-based businesses, forms are unavoidable. Even with the most comprehensive software, forms are sometimes necessary for collection and verification of data.
While many software vendors offer electronic form functionality, in most cases the information contained in those forms is not stored in the database. This means relying on PDFs or printed documents to access the data contained in the form.
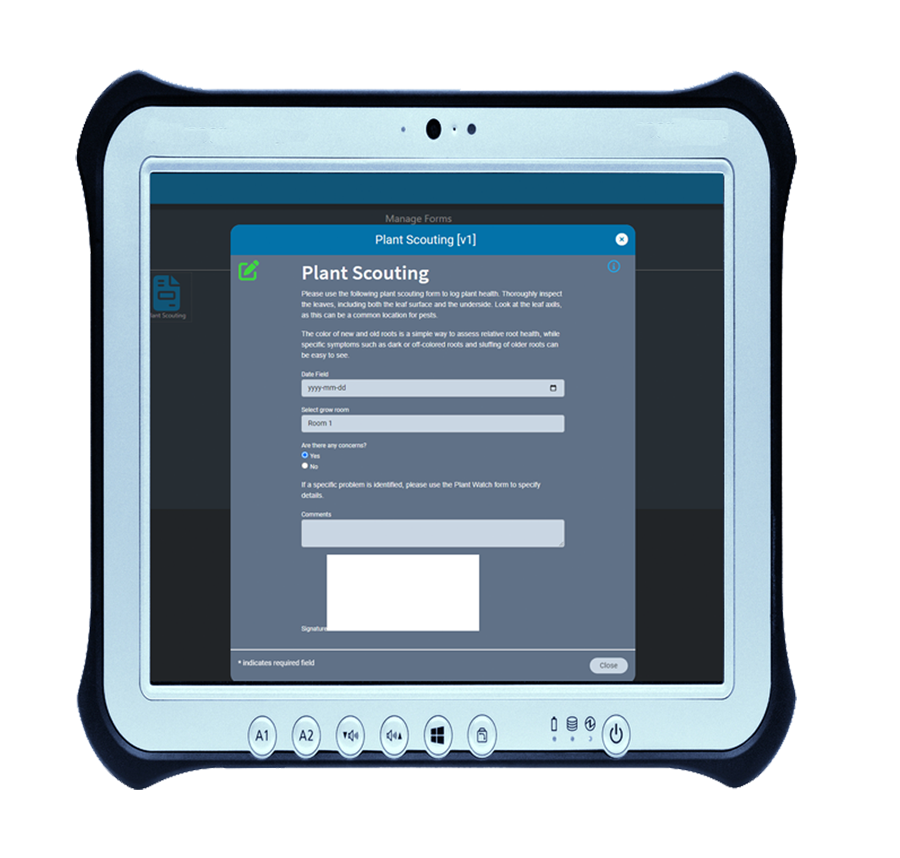
Our built-in form designer lets you create custom electronic forms that integrate with AirMed’s system data-sources. All fields in electronic forms created using the form designer can be stored in the AirMed database. There is no need to refer to physical documents or PDFs.
Using AirMed, you can automate workflows using custom e-forms to prevent the need for paper-based documentation. Forms can be linked to processes such as plant scouting, nutrient checks, room sanitation, and shop floor data collection. Using our form designer, you can employ drop-down picklists, date & time, checkboxes, radio groups, custom fields and more. AirMed lets you apply auto-complete, file upload, hidden input and electronic signatures to your forms.
All the data is electronically accessible and even reportable.
Form data collection doesn’t get any easier than that.
For more information visit our AirMed 5 page.
Workforce Management in AirMed 5
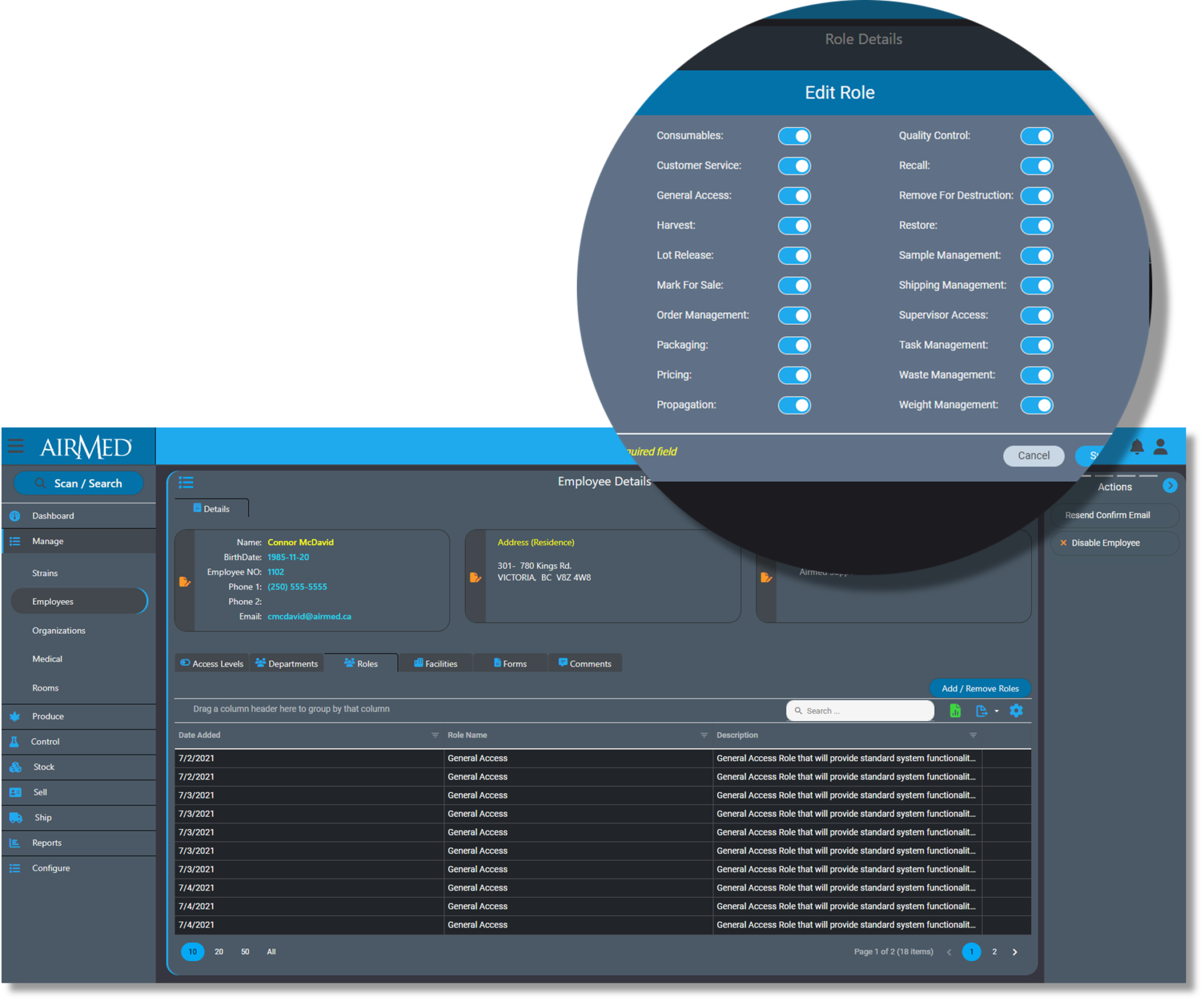
AirMed simplifies compliance by tracking and logging every action and ensures audit readiness with reports and analytics.
Employees only have access to functions and data that relates to their job responsibilities. System Administrators can only access data through approved computers utilizing a VPN connection.
And AirMed offers licensing by facility size rather than by individual user to encourage producers to configure individual accounts for every worker.
Our system improves quality management by allowing producers to identify gaps in employee knowledge and skills.
To see for yourself what AirMed can do, click the Request Demo button at the top of the page.
For more information visit our Software page.
Implementing AirMed in Your Facility

As a cloud-based platform, AirMed does not require expensive dedicated hardware to operate. Producers are free to use any computing device that supports a web browser. As a result, set up consists mainly of configuring the software to meet your needs. The rest of the time involves learning to use the system and getting your employees up and running.
How AirMed is implemented within your organization depends on the areas of the software you’ll be using. The first step in any software implementation is a needs assessment to help identify exactly how the software will be used. Understanding the capabilities of AirMed and how it can work with your processes is one of the keys to successful implementation. Your AirMed implementation specialist will work with you to perform an assessment of your needs to determine which areas of the software should be configured.
We provide an implementation guide and a production checklist that you can go through with your AirMed Implementation Specialist. Together, you’ll review the functionality in AirMed and determine which areas and functions are needed for your business operations. AirMed includes the ability to disable navigation menus for areas that you won’t be utilizing to provide a streamlined interface for your workers.
When you have completed your assessment and training, the next step is to configure your Live Production environment. After you’ve set up and tested your system, it’s time to train other users (your workers) to use AirMed.
Once users have usernames and passwords, they can access the training resources in the AirMed Learn environment. Workers can take additional training at a later date to develop skills for different areas of the system — in fact, anyone with access to AirMed can use the Learn environment at any time to practice new workflows or refresh their knowledge. When employees have completed training, you can give them access to the system, so they can get to work.
With AirMed you can implement only the functionality you need right now. For example, you can start by implementing the AirMed Grow module, then when you expand your operation, you add modules that meet your current needs for performing extractions, packaging for provincial sales, selling to medical patients or whatever your business entails.
Working with your implementation specialist will help you see all the benefits of using AirMed and ensure that you are getting the most from our software.

For more information on how AirMed helps specific types of businesses, visit our Customers page or our Frequently Asked Questions page.
Ready to learn more about AirMed? Click the Request Demo button or call 1-877-313-2442.
ISO, SCC and the Canadian Cannabis Industry

ISO, the International Organization for Standardization, is an independent, non-governmental international organization with a membership of 170 national standards bodies.
Through its members, ISO brings together experts to share knowledge and develop voluntary, consensus-based, market relevant International Standards that support innovation and provide solutions to global challenges.
In Canada, the ISO is represented by the Standards Council of Canada (SCC). A Crown corporation, SCC was established by an Act of Parliament in 1970 to foster and promote voluntary standardization in Canada. SCC is independent of government in its policies and operations, although it is financed partially by Parliamentary appropriation.
When Canada legalized cannabis in 2018, SCC set about creating standards for the new industry believing that standards are essential for an effectively regulated marijuana market. These standards were considered groundbreaking when published in October of 2022.
ISO IWA-37, “Safety, security and sustainability of cannabis facilities and operations” is available for purchase through ISO in three parts.
Part 1 (ISO IWA 37-1:2022) covers requirements for the safety of cannabis buildings, equipment and oil extraction operations. https://www.iso.org/standard/84023.html
Part 2 (ISO IWA 37-2:2022) covers requirements for the secure handling of cannabis and cannabis products. https://www.iso.org/standard/84024.html
Part 3 (ISO IWA 37-3:2022) covers good production practices (GPP). https://www.iso.org/standard/84025.html
Taken as a whole, these documents provide invaluable direction to legislative bodies and emerging companies and help to create a safe, legal market for adults who use cannabis.
For more information from SCC visit: https://www.scc.ca/en/news-events/news/2022/new-guidance-from-iso-international-workshop-safe-cannabis-production
For more information from ISO visit: https://www.iso.org/en/contents/news/2022/10/standards_safe_legal_cannabis.html
To learn more about how AirMed helps you meet these standards, visit our Compliance page.
If you’d like to learn about our quality management and GPP offerings or discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms.
AirMed Named Best Cannabis Management Software Company in Commercial Cannabis Awards

AirMed has been awarded the title of Best Cannabis Management Software Company by Global Health & Pharma magazine in the Commercial Cannabis Awards for 2023.
“We’re so proud to win this award,” said Justin Hearn, president and CEO of AirMed Canada Systems Inc. “This is the third time we’ve been recognized in GHP’s Commercial Cannabis Awards. But being named Best Cannabis Management Software Company is a true honour.”
The team at GHP wrote that the award “attests to the dedicated efforts of the whole team at AirMed Canada Systems Inc.!”
To determine the results, Global Health & Pharma’s judging panel and research team “consider the commitment, expertise, and innovation shown by nominees through nomination information, voting information, any supporting evidence that you have supplied, along with the results from their own extensive period of fact-checking and research.”
From all of us at AirMed, thank-you GHP for this tribute.
See our award on the GHP website here: https://www.ghp-news.com/winners/airmed-canada-systems-inc-3/
Read about AirMed in a previous awards magazine here: https://www.ghp-news.com/issues/commercial-cannabis-awards-2021/10/
Learn more about the GHP Cannabis Awards here: https://www.ghp-news.com/awards/commercial-cannabis-awards/
If you’d like to learn more about AirMed and why it was given this honour, please visit our software page: AirMed Software
And drop by our Customers page to see how we are helping businesses just like yours: AirMed Customers
QMS-GPP Planning According to ISO:9000
Introduction
Quality management is the practice of ensuring consistency in products and services throughout your organization. A Quality Management System (QMS) helps your cannabis business provide customers with the best you can offer while mitigating risks using tools that support Health Canada’s Good Production Practices.
A Quality Management System-Good Production Practices Plan provides an overview of the quality management system that an organization has in place. Although there are many standards in the world, ISO:9000 is one of the most respected. And according to ISO:9000 standards, the plan must contain the following.
Quality Policy (ISO:9000 clause 5.2): A quality statement can be derived from a mission statement and/or vision statement, but should explain the organization’s commitment to quality
Quality Objectives (ISO:9000 clause 6.2): These can be the organization’s objectives from a business plan, again, as long as they contain a commitment to quality
Criteria for Evaluation and Selection of Suppliers: As quality management and good production practices are often dependent on supplies and equipment that come from other organizations, organizations need to have a criteria in place for evaluating their suppliers to ensure that they select suppliers that meet QMS and GPP standards
Scope of the QMS: This is a list of all SOPs with brief descriptions/purposes
Quality Product Statement
ISO:9000 requires your organization’s quality policy to be appropriate to your organization’s strategic direction and operational direction (context).
Your organization must understand and identify all the influences that affect its business and ensure that the strategy and direction takes quality into consideration. Your organization will need to review its current quality policy regularly to ensure that any changes in context, interested parties or other requirements are reflected, and to determine whether your organization’s objectives are affected. (ISO 9001:2015 – 6.2.1a.)
Following is an example.
Company ABC produces cannabis products for distribution in Canada according to the regulations in the Cannabis Act. The Company has developed its production system through experience and its aim is to achieve a high standard of production and products to its customers.
It is the policy of Company ABC to provide the customer with goods to the agreed requirement in accordance with the details and price.
The Directors, Management and Staff are responsible for Quality Control through the Quality Management System seeking improvement by constant review, with suppliers and sub-contractors being encouraged to co-operate. The Company is committed to achieving customer satisfaction by the use of quality procedures which will be operated to meet or exceed the requirements of [the Cannabis Act and/or ISO 9001 or other quality system].
Quality Objectives
The quality objectives should act as a driver for continual improvement. To meet quality standards, your organization will be required to ensure that you continually improve products and services to meet customer requirements and to measure effectiveness of the processes responsible.
Following is an example.
Company ABC strives to be the best provider of cannabis products in Canada. Through the use of this guiding principle, everyone in Company ABC is accountable for fully satisfying our customers and authorities by meeting or exceeding their needs and expectations with best-in-class production practices. Our goal is 100% customer satisfaction and compliance 100% of the time.
Our Quality Policy is defined and strongly driven by the following objectives:
1. Meet all compliance requirements for all levels of governments and regulatory agencies
2. Build a mutually profitable relationship with our customers, ensuring their long-term success, through the understanding of their needs and the needs of their customers as well
3. Achieve our commitments for quality, cost, and schedule
4. Use of best preventive practices at all levels and ensure reliable risk management
5. Drive continual improvement and innovation based upon efficient business processes, well-defined measurements, best practices, and customer surveys
6. Develop staff competencies, creativity, empowerment and accountability through appropriate development programs and show strong management involvement and commitment
Evaluation and Selection of Suppliers
Supplier evaluation is a system for recording and ranking the performance of a supplier in terms of a variety of criteria and is a must in ISO:9000. A process of vendor rating is essential to effective purchasing. While there is no one right system for supplier evaluation and selection process, the overall objective is to reduce risk and maximize overall value to the purchaser.
Criteria
There are eight common supplier selection criteria:
1. Cost
2. Quality & Safety
3. Delivery
4. Service
5. Social Responsibility
6. Convenience/Simplicity
7. Risk
8. Agility
In the cannabis industry, you should also add a commitment to meeting compliance and/or helping their customers meet compliance.
Methods
There are many other methods of evaluation, and the organization should determine which is the best for its use.
Categorical systems typically use excellent, good, average, poor and so on.
Weighted systems rate on a scale from 1 to 10 or out of 100.
Hierarchical systems give values in relation to each item’s importance. The most important item is given the highest value.
Conclusion
Of course, a quality management system and good production practices plan is only as good as the processes that support it. Creating standard operating procedures and ensuring that all personnel follow them will give you the best chance of success.
For more information about ISO:9000 visit: ISO – ISO 9000 family — Quality management
For information on how AirMed helps you meet compliance, visit our Compliance page.
If you’d like to learn about our quality management and GPP offerings or discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms.
AirMed and FDA CFR 21

We are sometimes asked if AirMed meets FDA standards. First, please be aware that the FDA is a department of the US government. The specific portion of FDA regulations relevant to software such as AirMed is part CFR 21. As it is an American standard, Health Canada does not require CFR Part 21 compliance as part of the Cannabis Act Regulations. And while AirMed was designed for the Canadian cannabis industry and to comply with Health Canada regulations, AirMed does conform to CFR Part 21 with respect to electronic record keeping, audit trails, and electronic signatures.
Many agencies throughout the world are responsible for issuing and enforcing regulations that affect businesses. The regulations affecting software such as AirMed are typically those related to records management compliance. And while there are many regulatory agencies involved in records management, for the most part the regulations themselves are similar from country to country and agency to agency. The purpose of them, in general, is to ensure the security, confidentiality and authentication of electronic records.
The U.S. FDA regulates food, drugs, medical devices, biologics, animal feed and drugs, cosmetics and radiation-emitting products such as cell phones for the U.S.A. The FDA’s rules for manufacturing and distribution are designed to protect consumers and promote public health. In the U.S. Code of Federal Regulations (CFR), Title 21 deals with Food & Drugs. Until recently, the regulations in this title required paper records with handwritten signatures.
Back in 1997, part 11 of 21 CFR was enacted to cover the use of electronic records and electronic signatures. Commonly known as 21 CFR 11, this part defines the criteria “under which the agency considers electronic records, electronic signatures, and handwritten signatures executed to electronic records to be trustworthy, reliable, and generally equivalent to paper records and handwritten signatures executed on paper.”
Essentially, the concerns about using electronic records are that records may be lost in a system crash, the data may become corrupt or modifications may be made without proper authorization. In addition, since printed documents with hand-written signatures are recognized as legally binding on the signators, the agencies are looking for ways to make electronic records similarly binding on their owners. The regulations have been proposed to ensure that whenever an organization replaces printed documents with electronic data, there are checks and balances in place to ensure integrity of the electronic records so that they can be legally equivalent to printed records.
AirMed has a range of features that satisfy standards for security, authentication, validation and auditing as outlined in 21 CFR 11 and other regulations.
For more detailed information visit: Code of Federal Regulations (CFR) | FDA
For more information on how AirMed helps you meet compliance, visit our Compliance page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
FREE Seed-to-sale Software Buyer's Guide

Record keeping is an essential part of Health Canada’s compliance regulations. From the advent of legal medical marijuana in Canada, legal producers of cannabis have been required to track every seed, rooted plant, gram of waste material, final dried product, as well as interactions with customers and more. Due to the sheer volume of information, an electronic record-keeping system is the only practical way to manage the process. The software industry has responded to this need by creating seed-to-sale management software systems designed to help producers track their operations and report to Health Canada to meet compliance.
To help you through the process of purchasing seed-to-sale software in the Canadian Cannabis marketplace, we’ve produced a 20-page guide that answers the following questions:
- What is a seed-to-sale software solution and why do I need one?
- How do I choose one software platform over another?
- What should I be looking for in the software?
- How does the software fit into my business?
- How is my data stored and secured?
- What happens if regulations change?
To download this guide courtesy of AirMed, please visit the following page and complete the form. Once you submit the form, you’ll be able to download the guide.
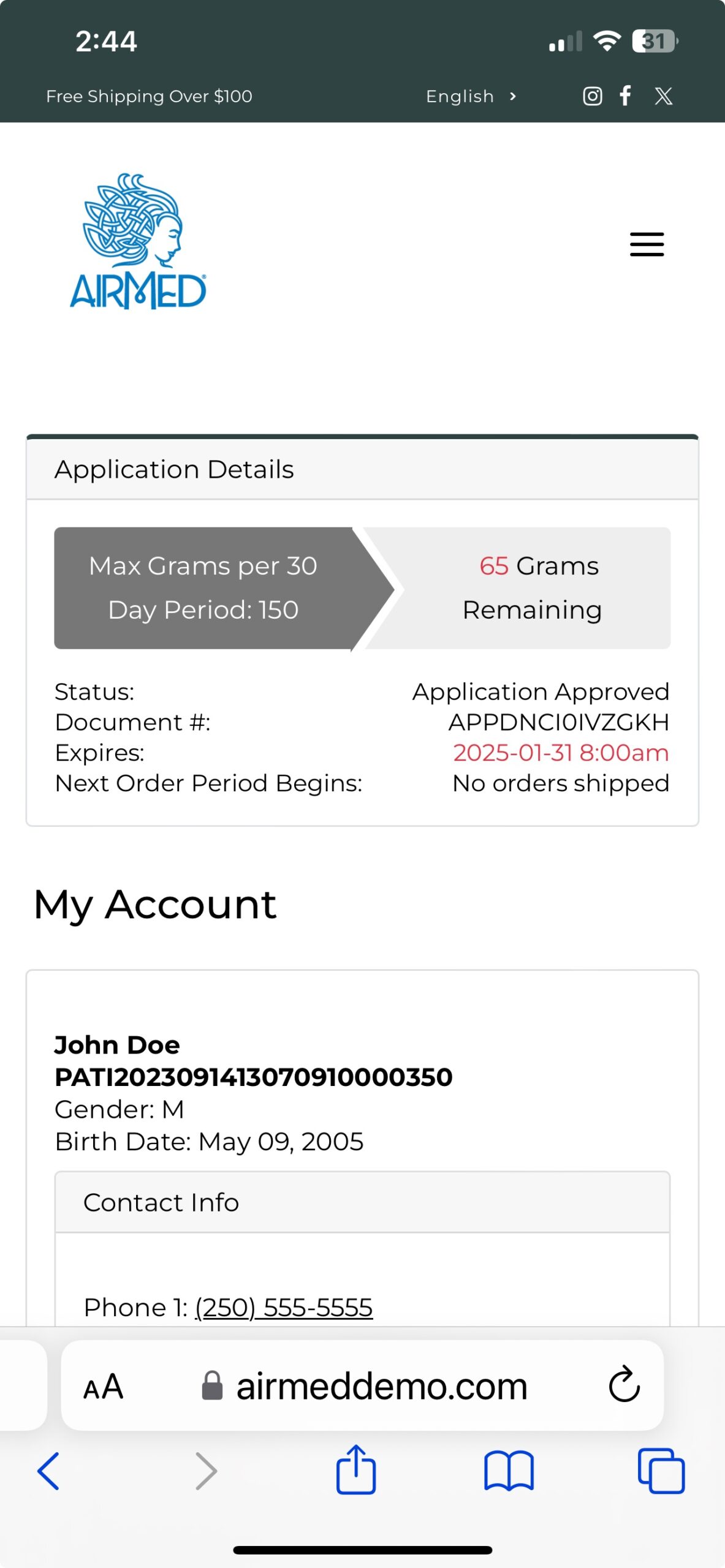
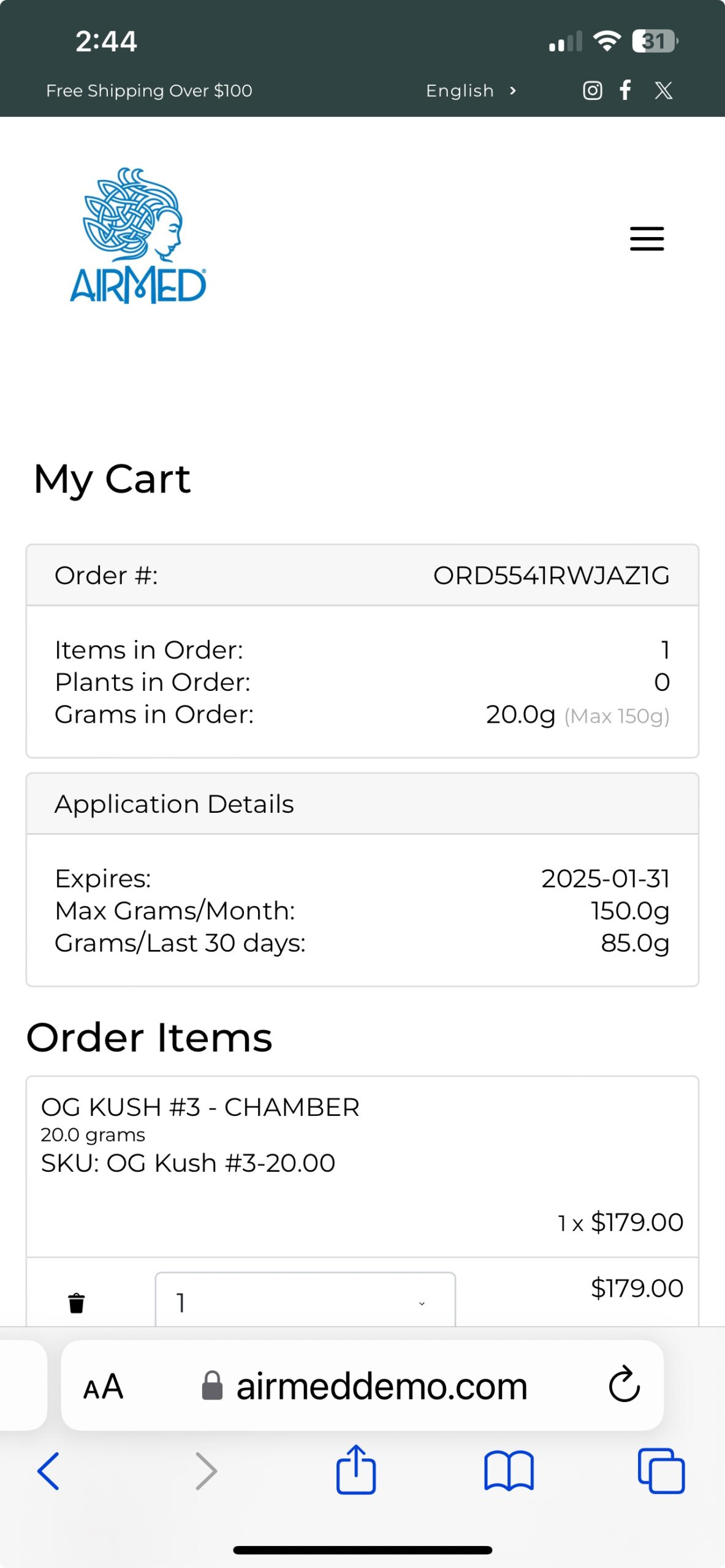
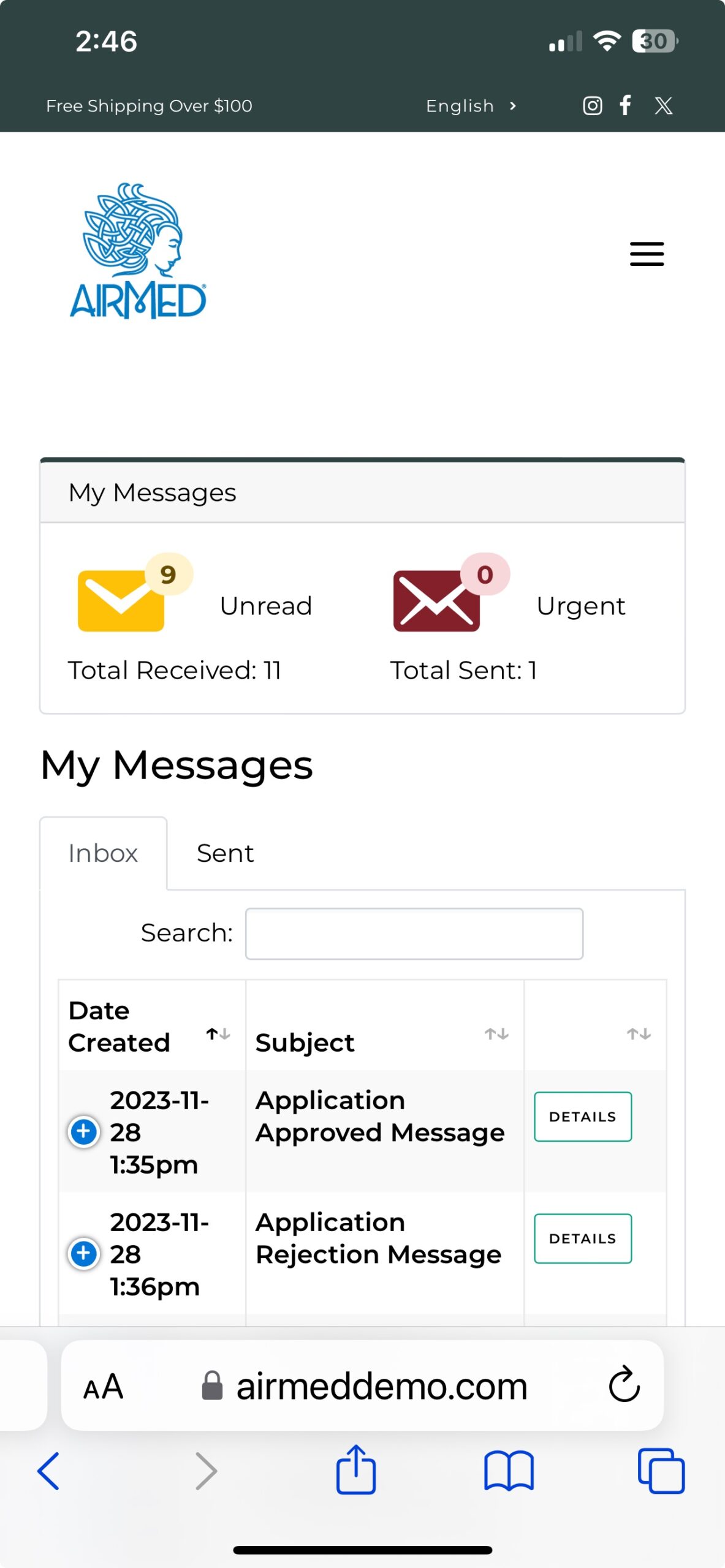
AirMed Plugin for Your WordPress Website
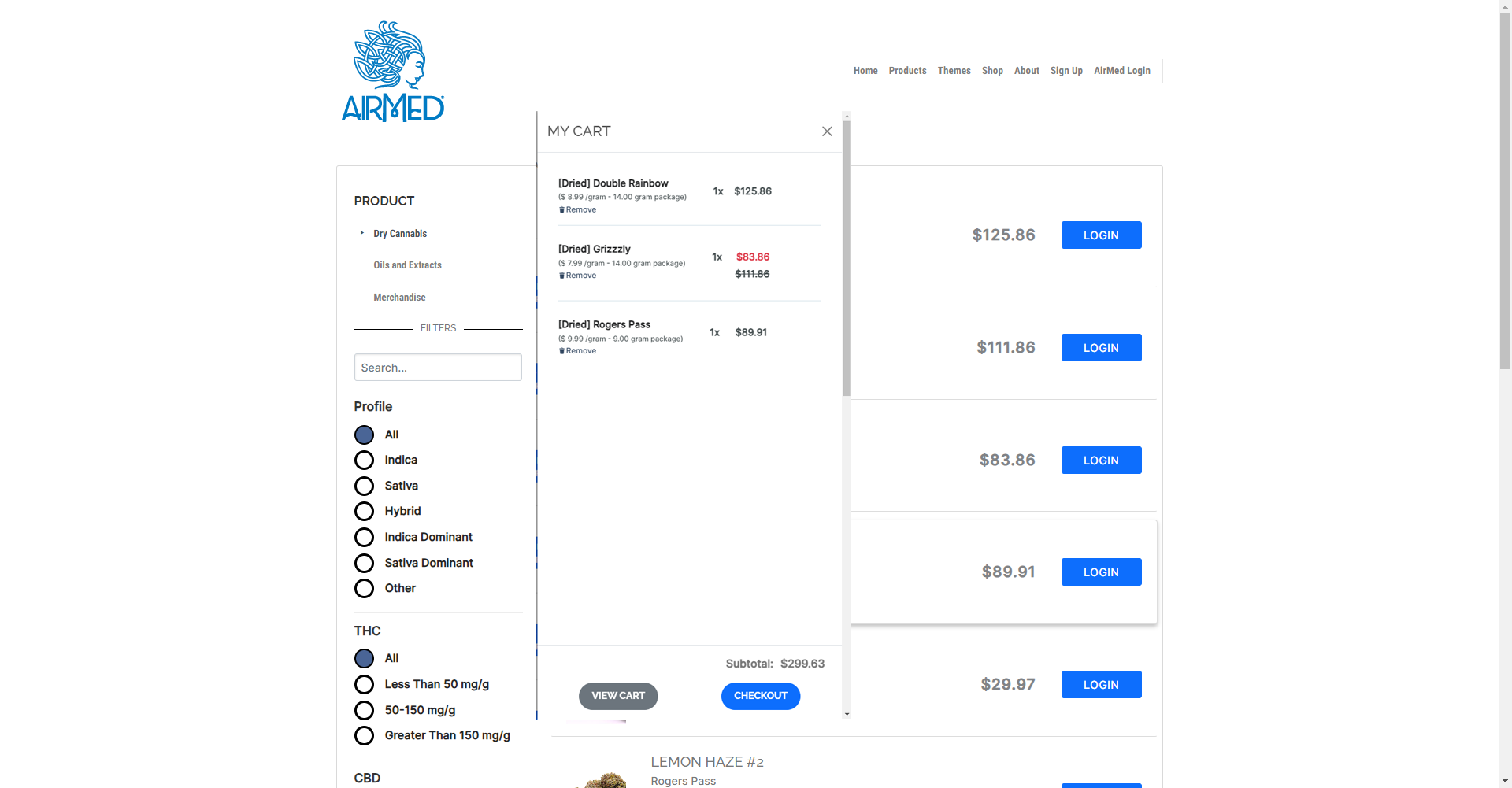
The AirMed WordPress plugin can pull information from your AirMed account to showcase your brands and products in an online catalog. Content stored in your AirMed database is published directly to your WordPress web pages.
Use the pre-designed theme options in your AirMed plugin settings to determine how your catalog will appear on your website.
View your choices in a visual interface installed on your WordPress dashboard. Save the settings to see the finished result on your web page.
Although the catalog appears on your website, all product details are stored in AirMed. Payments are processed by the merchant solution provider with no card data passing through AirMed. Patient information is encrypted by Canada’s most secure hosting facility. With support for monitored intrusion prevention and monitored firewall, your records have the highest level of protection.
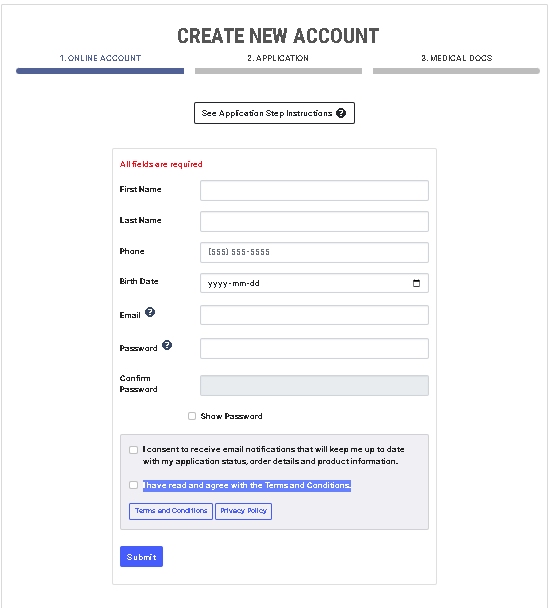
As an AirMed customer, you install a simple plugin onto your WordPress website. The plugin installs a visual interface in your WordPress dashboard.
Then add a snippet of shortcode to the page where you want your catalog to appear. The shortcode pulls directly from your AirMed database, which contains all your products complete with images, descriptions and pricing.
Customers interacting with the product catalog remain on your website throughout the shopping experience rather than being transferred to a portal site.
The WordPress plugin interfaces with our application programming interface (API) to embed information from the AirMed database directly onto your website.
Change the style, format, and position of catalog elements using the plugin settings and see how the elements will appear on your website before committing. When you find the combination you like, save your settings and refresh the web page.
For more information on these new features or to book a demo of AirMed to see them for yourself, click the Request Demo button at the top of the page or use any of the contact forms.
In the meantime visit our Intro to AirMed 5 page.
AirMed Named Most Innovative Cannabis Management Software Company

Innovation in Business magazine has named AirMed “Most Innovative Cannabis Management Software Company” in the Technology Innovator Awards for 2023.
This awards program celebrates the outstanding achievements of businesses in the dynamic world of technology. “Innovation in Business recognizes the paramount importance of acknowledging the remarkable contributions made by businesses in the ever-evolving technology sector.”
“We’re proud to receive this Technology Innovator Award,” said Justin Hearn, president and CEO of AirMed Canada Systems. “Innovation is one of the driving forces behind AirMed. Recognition like this is an acknowledgement of the hard work we put into anticipating and meeting our industry’s needs.”
On behalf of all the staff at AirMed, we’d like to thank Innovation in Business for honouring us with this award. We’d also like to thank our customers who motivate us to give them the best we can.
To read about our award at Innovation in Business visit:
https://www.innovationinbusiness.com/winners/airmed-canada-systems-inc/To read the media announcement about the Technology Innovators winners for 2023, visit: https://www.innovationinbusiness.com/innovation-in-business-announces-the-winners-of-the-technology-innovator-awards-2023/
To see what we’re doing that helped us win this award, visit our AirMed 5 page.
For more information about all our awards, visit our About page.
Important Cannabis Council of Canada Excise Tax Request

Cannabis Council of Canada surveyed licensed producers and has released their findings in a report that you can access here: https://cannabis-council.ca/files/advocacy/C3-Excise-survey.pdf
But that’s not the end of this story. In fact, it’s just the beginning. CCC announced that after bringing their excise tax survey results to Ottawa during the Grass on the Hill Summit last month, the Department of Finance wants to learn more.
“The Department of Finance would like to get a deeper understanding of how excise tax impacts License Holders’ financial positions, and how different rate models can help the long-term viability of our sector.”
This is where you come in.
CCC is asking Health Canada License Holders who pay excise tax to provide the Canadian government with a confidential look at their financials for 2022. Note that deadline to respond: November 30, 2023.
For details and to access the CCC excise model spreadsheet use the contact information below before the November 30 deadline!
Email CCC directly at hello @ cannabis-council.ca
If the email link doesn’t work, visit the Cannabis Council of Canada website for contact information: https://cannabis-council.ca/
Intro to AirMed5 Booklet

We’re so excited about the release of AirMed5 that we’ve created a booklet describing the new software in detail. This document covers all the features in the initial release.
- Re-imagined interface and user experience
- Fully customizable tables with custom columns
- Built-in report, label and e-form designers
- Business intelligence designer
- SOP library with built-in SOP editor
- Automated QMS incident management
- Task-based workflows with support for task templates and automated task scheduling
- Product plans
- Simplified packaging and shipping
To receive a PDF of this booklet by email, please send a request to sales @ airmed.ca or fill out one of our request demo contact forms.
In the meantime, visit our AirMed 5 web page.
Redesigned Order Screen in AirMed5

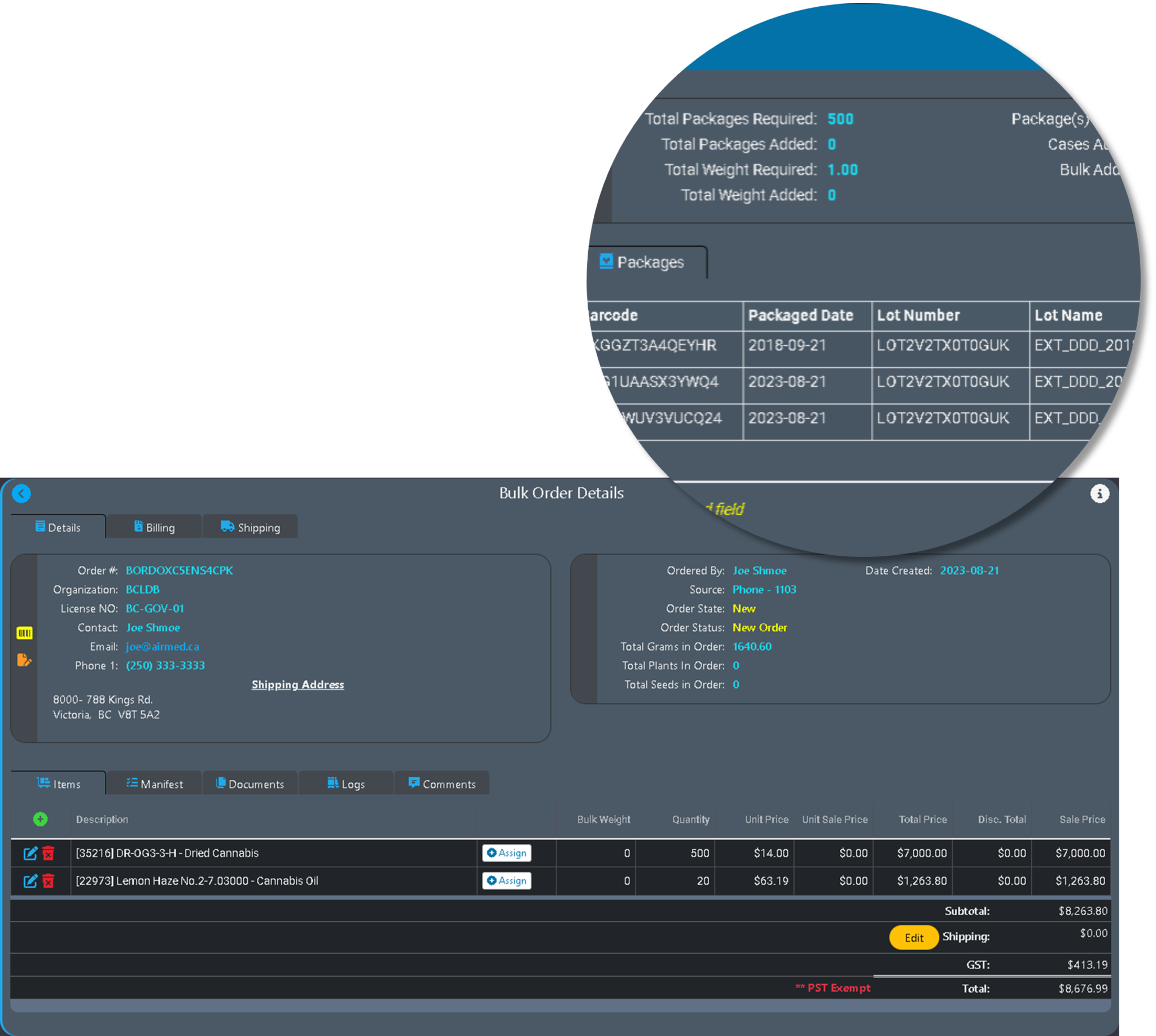
AirMed5 lets you manage order requests, order planning, order assembly, and shipping from one screen.
After releasing a planned order to production, verify order items in one step. Once you have entered the customer requested products and quantities, browse available inventory from multiple lots to plan the order fulfillment.
The new order processing in AirMed5 offers unparalleled ease while managing complex order requirements.
Manage products and different package types using the new Items table. Items and Item variants let you easily browse and manage products, different package sizes and available inventory.
Create new package runs and pack cases with streamlined workflows. Manage bulk items, recreational products, and medical products.
For more information on these new features or to book a demo of AirMed to see them for yourself, click the Request Demo button at the top of the page or use any of the contact forms.
In the meantime visit our Software page.
We’re streamlining so you can work faster
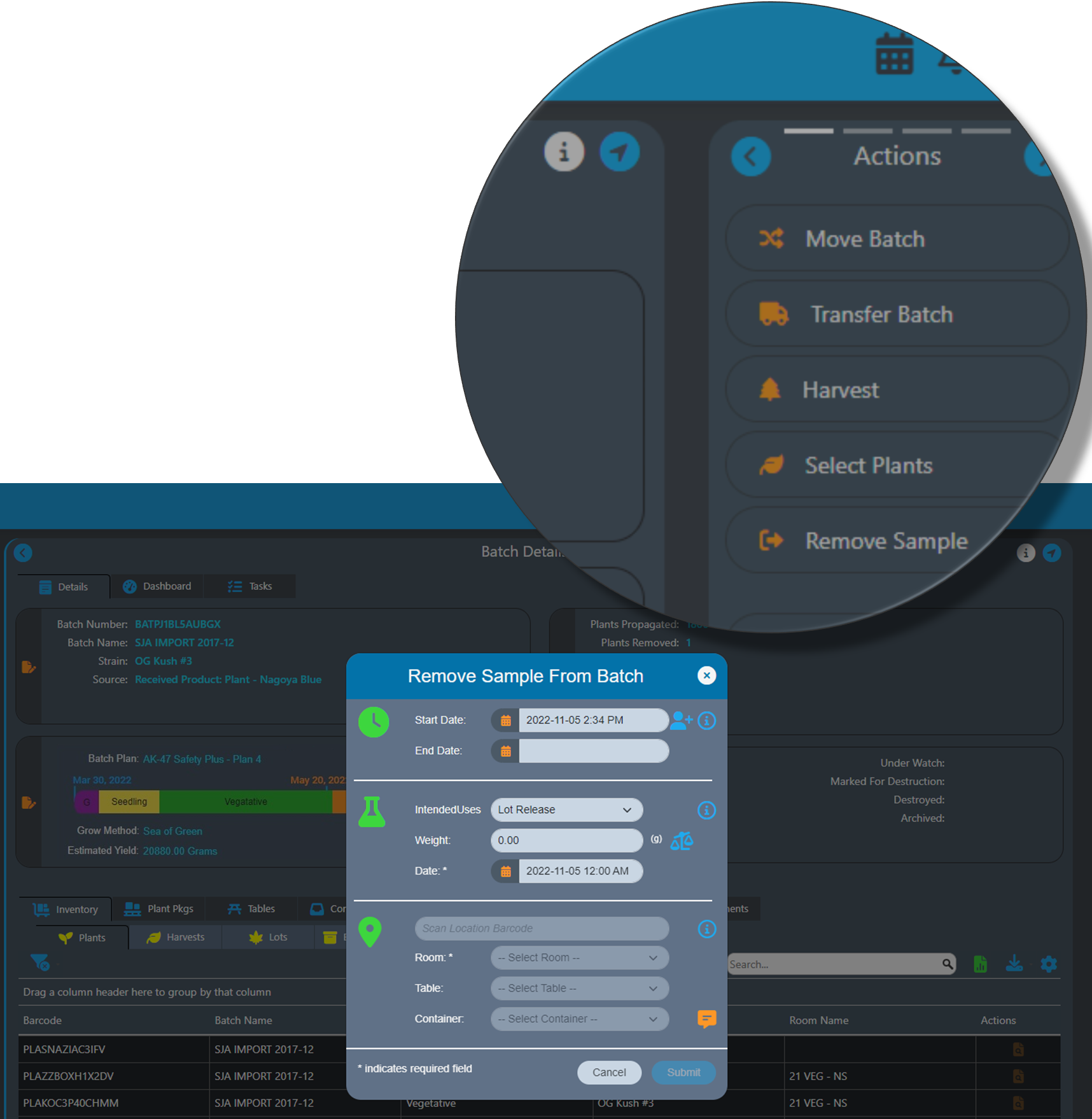
AirMed 5 streamlines processes so that you can work faster and more efficiently. Each action workflow has been redesigned to minimize the number of clicks required to complete the step.
Comments are available in a pop up, and lists can be auto populated by scanning a barcode. To select a location, it’s possible to scan the barcode for a room, table, or container, and all the pick-list values will be selected.
Smart input fields ensure erroneous data cannot be entered. If the database contains only a certain number of items, the system will not allow a higher number to be entered. Date fields are automatically restricted based on other data in the system so that it becomes impossible to back-date an item to a date before the item was recorded in the system.
Required fields in action dialogs are available on the first screen the user encounters.
And AirMed 5 extends the actions menu with a carousel to show Actions, Reports, SOPs, and Forms. The Report, SOP, and Form sections have customizable lists where users can select the items that are relevant to them.
For more information on these new features or to book a demo of AirMed to see them for yourself, click the Request Demo button at the top of the page or use any of the contact forms.
In the meantime visit our Software page.
Cannabis Prospect Interviews AirMed CEO Justin Hearn

Cannabis Prospect interviewed AirMed Canada Systems Inc. president and CEO Justin Hearn for an article published on the magazine’s website on May 15, 2023.
In the feature, titled “How AirMed is Helping Canadian Cannabis Producers Cultivate Success,” Hearn discussed the ways AirMed software is adapting to the changing market conditions.
“Our new AirMed 5 version focuses on streamlining data entry and improving quality management and profitability for cannabis operations… We’ve modelled the workflows on commonly accepted manufacturing procedures and terminology incorporating task-based work orders that let organizations precisely track the costs incurred for running their facilities.”
Hearn goes on to say, “AirMed is adapting its software to support increased insight as well as new strategies to boost efficiency, reduce costs, and improve the bottom line.”
Read the full interview here: https://cannabisproonline.com/article/
Download a PDF of this article: Cannabis Prospect Justin Hearn Interview PDF
For more information on AirMed 5, visit: https://airmedcloud.com/intro-airmed-5/
Multi-tier Approvals in AirMed5
The upcoming release of AirMed5 includes the option to record eSignatures and lets you create an approval workflow with one or more signatures required for a given activity.
Approval workflows can include a single signature or one or more unique signatures plus optional witnesses. When an individual is tasked with electronically signing for an approval, a notification is sent through the AirMed internal messaging system. Within the message is a quick link to the location where the eSignature is required.
For more information on these new features or to book a demo of AirMed to see them for yourself, click the Request Demo button at the top of the page or use any of the contact forms.
In the meantime visit: https://airmedcloud.com/intro-airmed-5/