AirMed Nominated for Two 2022 O'Cannabiz Awards

We’ve been nominated in the O’Cannabiz Awards as Best Software and Best Innovative Technology!
Hosted by the producers of the O’Cannabiz Conference & Expo, the awards program is designed to recognize excellence and innovation in our industry.
Between now and May 14, 2022, anyone can vote on which nominees will win in the O’Cannabiz Awards. To vote you need to register for free on the O’Cannabiz website. Once registered, you can go back to vote any time before the deadline, but registered voters can only vote once in each category (see list below).
An awards gala will be held during the O’Cannabiz Conference & Expo on June 1st, 2022 in Toronto to honour cannabis professionals and companies.
Please vote for AirMed in the Best Software and the Best Innovative Technology categories by registering at:
https://awards.ocannabiz.com/To learn more about the conference & expo, visit: https://ocannabiz.com/
O’Cannabiz Awards categories
Attorney of the Year
Best Activist/Advocate of the Year
Best Budtender
Best Business Consultancy
Best Cannabis Clinic
Best Cannabis Nutrients Product
Best Customer Service
Best Disinfection and Pest Control Services
Best Engineering Firm
Best Extraction Equipment
Best Extraction Facility/Toll Processing
Best Growing Medium
Best Home Grow Package
Best HVAC & Ventilation Systems
Best Hydroponics System
Best Innovative Technology
Best Lab Equipment
Best Lab Testing Facilities
Best Lighting Systems
Best Marketing Campaign
Best new Start-Up
Best News Source
Best Packaging Design
Best Packaging Equipment
Best POS/CRM Software
Best PR/Advertising Agency
Best Retail Chain
Best Retail Interior Design
Best Security Service
Best Seedbank
Best Software
Best Storefront Branding
Best Supplier/Distributor
Best Top Retail Store (Single Location)
Best Trimming Equipment
Best Water Filtration System
Brand of the Year
Cannabis Person of the Year
Favourite Accessory
Favourite Capsules, Extracts, Concentrates
Favourite CBD & Topicals
Favourite Edible Product
Favourite Flower
Favourite Infused Beverages
Favourite Product of the Year
Favourite Retail Store or Medical Clinic
Favourite Vaporizer
Green Deed(s) Award
Licensed Producer of the Year
Medical Cannabis Professional of the Year
Outstanding Educator or Institution of the Year
Scientific Researcher or Facility
Social Media Influencer of the Year
New reports in AirMed keep pace with marketplace

AirMed has the most comprehensive reporting in any cannabis management system. To ensure that our reporting keeps pace with changes in regulations and the cannabis marketplace, we regularly update our reporting system and add new reports.
Industry analysts and market researchers agree that edibles are the future of cannabis as consumer preferences shift from away from smoking.
Seattle-based data-analytics firm Headset examined sales from the US and Canada and reported “…overall edibles sales grew by more than 20%…”
According to a different report titled Cannabis Edible Products Market Report, “The aversion to smoking is one of the main factors that has led to the significant growth of cannabis-infused edibles over the past years.”
MJ Biz Daily reported that “The outlook for edibles is considered bullish among industry executives… Industry executives said consumers around 30-45 years old especially seem to be entering the cannabis market via edibles.”
To support the addition of edibles and topicals functionality in AirMed earlier this year, we added new CTLS data reporting. We also updated the existing CTLS Full Export function to include the totals from the main CTLS reports.
The release of edibles and topicals functionality in tandem with supporting CTLS reporting lets users manage entire edible and topical inventory data in AirMed and also export data into a pre-populated CTLS monthly report using the CTLS Full Export function. This vastly improves the inventory tracking capabilities for edibles and topicals and can save users valuable time in monthly reporting.
AirMed is working hard to support consumer trends so cannabis producers and processors can meet the needs of the marketplace.
The “Cannabis Edible Products Market Report” can be found at: https://www.researchandmarkets.com/reports/5529328/cannabis-infused-edible-products-market-growth
The Headset “Cannabis Product Trends” report can be found at: https://www.headset.io/industry-reports/the-most-popular-cannabis-product-trends-in-the-us-canada
Read more from MJ Biz Daily on the edibles market here: https://mjbizdaily.com/led-by-gummies-edibles-keep-pace-with-growth-of-overall-us-marijuana-market/
To learn more about AirMed reporting visit: https://airmedcloud.com/software/#report
For a demo of AirMed’s reporting features, click the Request Demo button at the top of the page or use the contact form in the footer. You can also call us directly at 1-877-313-2442.
Quality Management and AirMed

Constantly innovating to help our clients, we have a host of features in development for our seed-to-sale software. One of the most significant improvements currently in development for AirMed is built-in Quality Management.
A Quality Management System (QMS) can help your organization mitigate regulatory risk by providing the tools you need to meet the requirements and standards for Health Canada’s Good Production Practices. But risk reduction is not the only benefit of QMS. You can also improve efficiency, provide consistent control over processes, and much more. As a result, we’re building a range of QMS features into AirMed including the following.
- Escalation and Tracking: Escalation and tracking will be available for use in any area of the facility such as plant growth incidents, extraction incidents, order and fulfillment incidents.
- Vendor Approval Process: A multi-level approval process will be supported for new vendors who supply products.
- Enhanced Received Product Management: New features are being added for received product quarantine, testing and release for use. The new functionality will support having certain received products undergo QA to ensure they match specifications. QA results can be uploaded to support this workflow.
- SOP Management: Standard Operating Procedures (SOP) are critical to QMS, and we will be adding SOP management for current and historical SOPs with auto-versioning to track changes in procedures.
- Electronic Signatures: Electronic signatures will be available for workflows such as destruction, sanitation, and processes that need approval from a responsible person in charge.
- Case Management for Plant Issues: With new support for both SMS and MMS notifications, a grow tech concerned about a plant will be able to send a photo to the head grower for instant review.
We’re working hard to meet shifting customer needs and market demands in the Canadian cannabis industry, turning your feedback into important product advancements. AirMed seed-to-sale business solutions go beyond compliance to help licensed producers cultivate success.
If you’d like to read about Canada’s Good Production Practices for Cannabis, visit https://www.canada.ca/en/health-canada/services/cannabis-regulations-licensed-producers/good-production-practices-guide.html
For more information about AirMed visit our software page.
If you have specific questions or would like a free demo of AirMed, please contact an AirMed sales representative by filling out one of our contact forms or calling 1-877-313-2442.
Software Validation for Cannabis Processors

One of the challenges for cannabis processers is that regulations require them to validate their extraction practices. In many cases, those practices involve the use of software. Therefore, any software used in relation to production must be validated along with the practices.
As tracking production reliability is an important part of meeting compliance, most tracking involves the use of software.
Software vendors are responsible for ensuring the software the sell works as described. But, validating that software is the responsibility of the processor. Why? Because every organization operates differently, and validation must be done in relation to actual production practices.
If you are using AirMed as your system-of-record, you will need to validate our software as it is used in your facility.
AirMed helps you through the validation process by providing test scripts that can be used to validate each task in the workflows that relate to extraction processes. These test scripts show what the software was designed to do, how the software should be used for that specific task and what the end result should be.
We can provide you with a validation report outlining how we meet software vendor compliance. Our validation report details how our software complies with security, privacy and accessibility requirements. AirMed software has been designed to not only meet compliance with Canada’s Cannabis Act but also to support and comply with Federal and Provincial privacy legislation and regulations (PIPEDA, PIPA, PHIPA, PHIA). AirMed also meets the standards outlined in the US FDA 21 CFR Part 11, which many other specifications are based on.
Our internal procedures have been through an external vendor audit for the pharmaceutical industry and passed with no observations.
If you’d like to learn more about good production practices for cannabis in Canada, the Government of Canada has published GPP guidance information here: https://www.canada.ca/en/health-canada/services/cannabis-regulations-licensed-producers/good-production-practices-guide/guidance-document.html
For more information on how AirMed helps you meet compliance, visit our Compliance page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
Managing Extracts, Edibles & Topicals in AirMed

For those wishing to process extracts, edibles and topicals, AirMed offers comprehensive features dedicated to these classes of cannabis.
Extracts, edibles and topicals are categorized as special classes of cannabis due to the unique public health and safety risks they present. As a result, these cannabis products have specific requirements pertaining to their formulation, production and composition.
Health Canada licensed processors have a broad base of data management needs to maintain compliance and accurately complete the monthly CTLS report. AirMed has developed a full range of functionality to meet those needs.
As with all elements of our system, comprehensive tracking and reporting is available. AirMed tracks and reports on every aspect of processing with specific fields for managing extracts, edibles, and topicals as well as their materials and lots.
Access to the AirMed software functionality that manages these cannabis classes is controlled on a user-by-user basis. Workforce management in our software utilizes configurable departments and job roles to control which workflows workers are authorized to use. You determine who is given rights to these production areas based on the needs of assigned job roles.
The AirMed cannabis record management system has separate production sections for extracts, edibles and topicals with workflows designed specifically to meet their requirements
AirMed offers workflows for storing extractions after processing for further processing or for blending with other materials and more. Weights are tracked to five decimal places for precision and reporting.
Packaged products can be sold individually to medical clients or as packaged products to recreational users.
Packaging features in AirMed include the ability to create discrete units such as edible gummies or gel caps, which can be recorded individually in groups or as bulk packages.
Packaging of discrete units can be one discrete unit in a package, multiple discrete units in a package, or bulk discrete units in a bulk package for either storage or to sell as bulk units to another licensed producer.
All labels are produced and created from within AirMed and can be customized to your needs.
To facilitate the sale of products to provincial warehouses, AirMed offers GS1 barcoding and configuration for tracking product cases and master cases.
Inventory management in AirMed is designed to provide a centralized view of your current stock levels of all products to help control costs, manage planning, and forecast with extensive reporting tools.
Shipping is easy with built-in functionality and integration with Purolator services.
Each field related to extractions, packaging, discrete units, samples, and order fulfilment is automatically included by AirMed in Health Canada CTLS and CRA monthly reporting.
With more stringent requirements for these cannabis classes comes a greater need for control and risk mitigation. Quality management (QMS) helps you provide the finest you can offer while reducing risks.
To give our clients the best chance of cultivating success, we’re building a range of QMS features into AirMed to support Good Production Practices (GPP) and a Preventive Control Program (PCP).
No other cannabis record management system offers the breadth of functionality, ease of use, and comprehensive features to cover all classes of cannabis and help you control cost.
- Dried cannabis
- Fresh cannabis
- Cannabis plants
- Cannabis plant seeds
- Edible cannabis
- Cannabis extracts
- Cannabis topicals
To read about the Health Canada requirements related to processing cannabis extractions, edibles and topicals, read the guidelines at: Government of Canada.
For more information about the features in AirMed, visit our Software page.
To schedule a free demo of AirMed, please use the button at the top of the screen or fill out any of the contact forms.
And if you’d like to learn more about any of the features mentioned, give us a call at 1-877-313-2442.
Role-based Management in AirMed
Role-based management in AirMed helps control employee access to the software system.
Actions within AirMed are linked to roles. When you assign a role to an employee, you allow access to those actions.
Employees can be assigned to the roles that correspond to the requirements of their jobs. Employees not assigned to specific roles are prevented from accessing the associated workflows.
For example, if a staff member is new and working in cultivation, you can create a role that only provides access to certain actions in the Source Material, Batch, and Lot Details screens. All other action items will not be visible. This lets you restrict access to parts of the system, based on a user’s job function within your organization.
AirMed has several roles defined by default, but you can create as many roles as required. You can also edit or rename existing roles to match your organization’s workflows. Within each role you can specify which actions are associated.
For more information about how roles can help you manage employee access, contact us today by using one of the contact forms or by calling 1-877-313-2442.
For more information on how AirMed helps you business, visit our Software page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
AirMed still 100% Canadian owned

As US corporations buy up seed-to-sale software companies in Canada, AirMed is still 100% Canadian owned.
AirMed was created in 2014 to help Canadian licensed producers meet compliance at all levels of government. Continuously innovating since then, we believe that building a culture of quality is an important part of our customers’ success. This commitment to quality is why a growing list of cultivators, nurseries, processors, manufacturers, and dispensaries use AirMed.
We rely on industry requirements and customer feedback to drive AirMed development, rather than investor pressure. We encourage our users to tell us what matters most to them in a cannabis management system. This feedback helps guide us in adapting AirMed to improve efficiency, productivity and user experience.
Our goal is to provide a responsive solution that not only meets customer needs but anticipates them, regardless of the size or focus of your cannabis business. That has been our driving force for nearly eight years.
Contact AirMed for a demo today to see what homegrown can do for you. Call us at (877) 313-2442 or use the contact form in the footer of this page.
For more information about AirMed, visit our About page or our Frequently Asked Questions page. If you’d like to have a conversation about AirMed, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
Support for GS-1 Barcoding Standards in AirMed
AirMed software offers full functionality for multi-level packaging with layered barcoding that meets GS-1 barcode standards. This is the standard required to sell to provincial government distributors.
Licensed producers who use AirMed can assemble retail-ready individual product packages using a ‘master case’ system and identify and track products at each packaging level.
AirMed software offers full SKU and barcode management that supports GS-1 barcoding standards. Pricing can be set at the package or master case SKU, and custom pricing can be set for specific SKUs by customer. The software offers features for creating and processing the orders as well as fulfilling and shipping them to provincial agencies.
Our master case processing lets you use layered barcoding for individual containers through to cases of multiple containers. The AirMed track-and-trace system allows retail packages to be traced to the lot or batch and ultimately back to the original genetic material. And AirMed has a comprehensive multi-step recall process that completely automates recalls if they are ever needed.
To schedule a live demonstration of AirMed Cloud software, call us at 1-877- 313-2442 or click the Request Demo button at the top of the screen.
For more information on how AirMed helps your business, visit our Software page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
Cannabis in Canada: Get the Facts

Do you have questions about cannabis in Canada? Would you like to understand the laws that govern the Canadian cannabis industry? Are you considering applying for a license to cultivate, process or sell cannabis?
The federal government has a webpage that outlines what industry needs to know about cannabis in Canada. This information covers the basics related to doing business in the cannabis industry including:
Impact of the new law and regulations on industry
- Applying for or amending a federal licence
- Packaging and labelling requirements
- Cannabis Tracking and Licensing System requirements
- Compliance and enforcement under the Cannabis Act
- Prohibitions on promotions
- and more
The page provides links to relevant sections of the federal government website where you can find more information on each topic.
https://www.canada.ca/en/services/health/campaigns/cannabis/industry.html
For more information on how AirMed helps you meet compliance, visit our Compliance page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
CTLS Reporting in AirMed
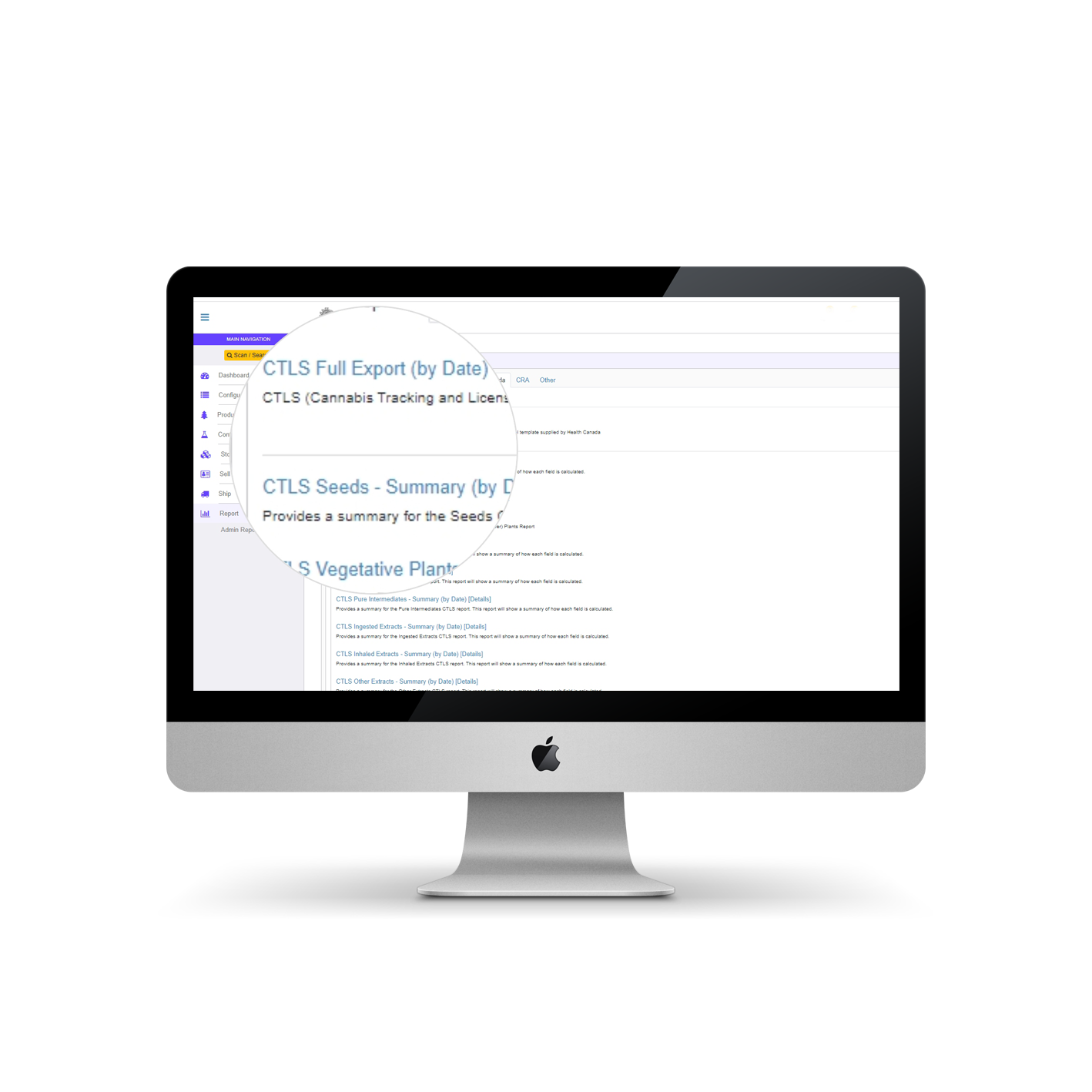
Always innovating to help our customers meet the regulatory challenges they face daily, AirMed offers a new automated system for CTLS reporting.
As mandated by the Cannabis Act, Health Canada requires that licensees submit a Cannabis Tracking and Licensing System (CTLS) report every month for every licensed site. This reporting applies to cultivators and processors (including micro, standard or nursery), as well as sellers of medical cannabis and provincial or territorial authorities, distributors and retailers.
Full Export
Using our new full export process, thousands of reported fields tracked by AirMed will automatically populate the standard spreadsheet template provided by Health Canada. Not only is the time required to complete monthly reports reduced, but the potential for human error often encountered in manual data entry is eliminated.
Summary Reports
AirMed also includes a suite of CTLS summary reports offering breakdowns of key fields with direct links to detailed reports. You can identify the values used to calculate a final total and also find the exact inventory records used to calculate those values, all from one page. Inventory validation has never been easier.
For more information or for a free demonstration of our automated CTLS reporting, please call 1-877-313-2442 or use the contact form linked at the top of the page. In the meantime, visit our Software page.
Quality Assurance in AirMed

AirMed provides licensed producers with a complete framework to establish and validate a quality assurance system.
The AirMed QA system is based on pre-defined North American and European pharmacopeia.
The system tracks testing results for the samples taken at any stage of production and enables shipping of samples to third-party QA labs. Results and certificate of analysis documents are logged and used to determine if the material the sample was derived from is acceptable for sale. Test results are verified by an authorized QA person and a passed or failed status is assigned to each data point. The QA results are maintained in the system and can be referenced at any point to review the values.
From reviewing non-conformance to documenting and communicating findings, AirMed helps you maintain your desired level of quality. Tracking attention to detail for every process gives you total confidence in your product.
For more information about quality assurance in AirMed, visit our Software page. You can also schedule a live preview of the software by clicking the Request Demo button at the top of the screen. Or give us a call at 1-877-313-2442.
Reporting & Auditing in AirMed

AirMed software is specifically designed to meet or exceed Cannabis Act regulations with full audit support capabilities and comprehensive reporting. AirMed regularly passes Health Canada inspections. For more information visit our Compliance page.
AirMed is designed to support and comply with federal, provincial and local legislation. In fact, AirMed meets the USA’s FDA 21 CFR Part 11 regulations. Considered to be some of the most comprehensive electronic record keeping specifications in the world, they are the basis for the standards set by many countries.
AirMed pulls all the required information for all government reporting, whether monthly, quarterly, or annually. Reports are built in for Cannabis Licensing & Tracking System (CTLS) and Canada Revenue Agency (CRA). Supporting sub-reports provide the necessary validation detail. Export data to other systems or print reports to retain the required information outside of AirMed as archived printed or electronic records.
Every action/workflow in AirMed is auditable, and built-in audit reports & forms provide complete product traceability. Inventory, recall, destruction and other reports can be generated as needed. Authorized site auditors can be given access to perform inspections of records. AirMed retains all data indefinitely and lets you export or print reports to be held for required retention periods for audit purposes.
For more information about AirMed’s reporting and auditing features, schedule a free demo by clicking the Request Demo button at the top of the page or by using the contact form in the footer. You can also call us directly at 1-877-313-2442.
How Secure is AirMed Software?

AirMed seed-to-sale software is a Cloud-based platform hosted in a third-party, Tier 3, SSAE 18 SOC1/SOC2 compliant, hardened data center in Canada, and all data remains in Canada. The same data center is used by Canadian banks and government agencies to secure their web-based applications. All data in AirMed is protected by fully managed and monitored enterprise firewalls and intrusion detection/prevention systems.
All communication between the browser and the server is encrypted using SSL. Sensitive data is additionally encrypted while at rest in the database. When a barcode is scanned, the resulting code is input into a field within the web browser. This data is then encrypted and sent to the server for processing.
Administrators can configure access based on an employee security profile so that individual workers only have access to the functions and data that relates to their job responsibilities. Electronic signatures and multi-factor authentication ensure only authorized users can access information and complete tasks. Full audit trails for every record and every action in the system allows administrators to review changes anywhere at any time.
All data is backed-up both locally in the data centre for near-line recovery and to a secure offsite location in Canada for disaster recovery purposes.
To learn more about AirMed’s privacy and security, contact us today by using the contact form or by clicking the Request Demo button at the top of the screen. You can also give us a call at 1-877-313-2442.
AirMed Compliance Documents for Health Canada Cannabis License Applicants

The Cannabis Act requires that license applicants provide information on how they expect to meet certain compliance requirements.
We offer full support throughout your license application process and provide documentation that outlines how AirMed meets Health Canada requirements. If you plan to use AirMed for compliance after you are licensed, you submit the documents we supply as part of your application. We also partner with specialists in licensing applications and business management if you need more help.
For applicants who expect to use AirMed to help meet compliance, we provide two comprehensive documents designed for submission with license applications.
AirMed Record Keeping Compliance: This document provides an overview of how AirMed provides support to enable a licensed producer to be compliant with the record keeping & reporting requirements of the Cannabis Act and Regulations (CAR) and the former Canadian Access to Cannabis for Medical Purposes Regulations (ACMPR). This document is intended to assist compliance auditors and internal compliance leaders in assessing readiness in relation to CAR requirements, as well as security and privacy standards.
AirMed Good Practices Compliance: This document provides an overview of how AirMed provides support to enable a licensed producer to be compliant with the good practices requirements of the Cannabis Act and Regulations (CAR).
For more information or to purchase our compliance documents, please call 1-877-313-2442 or email info@airmed.ca. You can also fill out the contact form in the footer.
For more information on how AirMed helps you meet compliance, visit our Compliance page or our Frequently Asked Questions page.
AirMed Recognized as Underlying Technology Software for Cannabis Sector

In a recent article by venture capital company Canopy Rivers, AirMed was recognized as one of the foundation vendors of seed-to-sale software. In a ‘market insights” post, Ben Futoriansky, an Associate in Business Development at Canopy Rivers, described specialized cannabis technology suppliers as “picks & shovels” for the sector.
“The sector-agnostic use case of many technology-driven innovations has led to software becoming the foundation in various industries. Following decades of prohibition and patchwork regulations, many established software solutions have not yet been applied to the cannabis sector. This has paved the way for specialized cannabis…”
To read the full article, visit Canopy Rivers.
Why you need seed-to-sale software for your cannabis business
 Introduction
Introduction
Seed-to-sale software tracks plant production from reproduction through growth, harvesting, drying, packaging, sales and distribution. Health Canada mandates record keeping for every part of the process, and Canadian seed-to-sale software applications focus on compliance built on Health Canada’s regulations.
Record keeping is an essential part of Health Canada’s compliance regulations. From the advent of legal medical marijuana in Canada, legal producers of cannabis have been required to track every seed, rooted plant, gram of waste material, final dried product, as well as interactions with customers. Due to the sheer volume of information, an electronic record-keeping system is the only practical way to manage the process. The software industry has responded to this need by creating seed-to-sale management software systems designed to help producers track their operations and report to Health Canada to meet compliance.
As a Health Canada applicant, you must specify the name of the software system you plan to use for record keeping and provide a summary of how the software program meets Health Canada’s requirements. As a result, you will need to make at least preliminary decisions into seed-to-sale management software early on in your application process.
This might seem like one more challenge along your path to becoming a licensed producer, but in reality, seed-to-sale software is designed to help you be successful.
Seed-to-sale software platforms should provide the electronic record keeping required to not only for you to meet compliance, but also to help you be successful in your business. From greenhouse to warehouse to customer, your software system should help you at every stage of your operation. Functionality for genetics tracking, inventory management, quality control, shipping & receiving, point-of-sale ecommerce, compliance auditing & reporting, and customer relationship management can provide you with full business administration.
Licensing
To do business in the cannabis industry in Canada, you must meet Canada’s licensing requirements. Currently there are several types of licenses for those wishing to carry out cannabis-related business in Canada.
- Standard Cultivation
- Micro-cultivation
- Nursery
- Standard Processing
- Micro-processing
- Sale
- Analytical Testing
- Research
Regardless of which class of license you get, you will be responsible for complying with all the regulations that apply from the various levels of government.
The Laws
The Cannabis Act
Formerly called the ACMPR, this act covers all aspects of cannabis in Canada from growing through processing to selling and more. It is the responsibility of the licensee/applicant to understand and meet all the requirements that apply to their business.
Other Federal Acts and Regulations
As a licensee or applicant, you are responsible for complying with requirements of other Canadian acts and regulations such as the Food and Drugs Act (FDA), the Pest Control Products Act, the Fertilizer Act, among others.
The Canada Revenue Agency
You’ll also need to meet requirements of the Canada Revenue Agency depending on which activities will be conducted with cannabis.
Provincial or Territorial Legislation
As a licensee or applicant, you’ll be responsible for complying with all applicable provincial or territorial laws and regulations (environmental laws, for example).
Municipal By-Laws
As well, you’ll need to deal with municipal by-laws such as zoning and building permits.
How can seed-to-sale software help?
From the advent of legal medical marijuana in Canada, legal producers of cannabis have been required to track every seed, plant, gram of waste material, final product, and more. Due to the sheer volume of information, an electronic record-keeping system is the only practical way to manage the process.
The software industry has responded to this need by creating seed-to-sale management software designed to help businesses meet compliance. Seed-to-sale software tracks growth, harvesting, processing, packaging, sales and distribution.
And some seed-to-sale software systems go beyond compliance to help businesses cultivate success with features and functionality to help fine-tune propagation, growth cycles, harvesting, production, derivatives, inventory, staffing, and product sales. AirMed is one of those. Wherever your business fits in the seed-to-sale supply chain, AirMed can help with both legal compliance and business acumen. For a free demo, call 877-313-2442, email info @ airmed.ca or fill out the form on our contact page.
For information on cannabis licensing in Canada visit: Cannabis Licensing Application Guide
For more information on purchasing seed-to-sale software, fill out a form to download our complete buyer’s guide: Seed-to-sale Software Buyer’s Guide
New AirMed version lays groundwork for October 17 Cannabis Act changes

A new version of AirMed seed-to-sale software was released October 16, 2019. This is a substantial build that lays the groundwork for the October 17 changes to the Cannabis Act and CTLS reporting.
The new version offers significant updates to the Received Products section of the cloud-based application including the ability to receive into the facility any type of product (edibles are coming soon) either bulk or packaged. The system now allows product to be transferred to a processor and packaged up, then the packaged material can be received back into the facility.
For received products, users now have the ability to add items to an existing lot, back-date inventory adds, and update finished/unfinished designation for new Health Canada regulations. We’ve also added an extraction material type category for new Cannabis Tracking and Licensing System (CTLS) reporting requirements, plus the ability to move all plants in batch and print a timeline report for batch details.
If you are a current AirMed customer, please explore the new features in your sandbox service, and let us know if you have any questions by contacting your customer service representative.
To learn more about the Cannabis Act in Canada visit: What you need to know about cannabis – Canada.ca

For more information on how AirMed helps your cannabis business, visit our Software page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
AirMed supports wholesaling to provincial distributors

AirMed Cloud seed-to-sale software supports wholesale selling and shipping from licensed producers to other LPs and from LPs to provincial distribution centres.
As a cannabis management system, our software offers full functionality for multi-level packaging with layered barcoding that meets GS-1 barcode standards. LPs using AirMed can assemble retail-ready individual product packages using a ‘master case’ system and identify and track products at each packaging level.
“Our master case processing lets you use layered barcoding for individual containers through to cases of multiple containers,” noted Justin Hearn, President and CEO of AirMed Canada Systems Inc. “AirMed’s track-and-trace system allows retail packages to be traced to the lot or batch and ultimately back to the original genetic material. And AirMed’s comprehensive multi-step recall process completely automates recalls if they are ever needed.”

AirMed software offers full SKU and barcode management that supports GS-1 barcoding standards. Pricing can be set at the package or master case SKU, and custom pricing can be set for specific SKUs by customer. The software offers features for creating and processing the orders as well as fulfilling and shipping them to provincial agencies.

“We’re proud to be supporting our customers with full master case inventory control, GS1 integration, comprehensive wholesale order management, efficient fulfillment and tracking, and compliance reporting,” said Hearn. “AirMed’s wholesale distribution module is helping LPs produce, prepare, sell and ship hundreds of thousands of products to the recreational market right across Canada.”
For more information on how AirMed helps your business, visit our Software page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
ACMPR and the licensed cannabis producer

What does ACMPR cover?
For the cannabis industry in Canada, the most critical compliance implications are those covered by the Access to Cannabis for Medical Purposes Regulations. This legislation covers every aspect of marijuana production and sale. Overseen by Health Canada, these regulations must be adhered to at every stage of cannabis business from propagation through to sale. As of August 24, 2016, the Access to Cannabis for Medical Purposes Regulations (ACMPR) replaced the previous Marihuana for Medical Purposes Regulations (MMPR).
ACMPR is designed to provide an immediate solution for Canada to meet legal requirements. But Health Canada is continuously evaluating how a system of medical access to cannabis should function alongside the government’s commitment to legalize, strictly regulate and restrict access to cannabis. As a result, laws and regulations may change.
Currently, the legislation contains four parts.
Part 1 sets out a framework for commercial production by licensed producers responsible for the production and distribution of quality-controlled fresh or dried marijuana or cannabis oil or starting materials (i.e., seeds and plants) in secure and sanitary conditions.
Part 2 sets out provisions for individuals to produce a limited amount of cannabis for their own medical purposes or to designate someone to produce it for them.
Parts 3 and 4 include transitional provisions, consequential amendments to other regulations, and provisions repealing the MMPR.
All those wishing to produce cannabis in Canada must apply to Health Canada to become a licensed producer under the ACMPR legislation, and all licensed producers MUST comply with the ACMPR regulations.
What are the specific ACMPR regulations that affect software and record keeping?
The ACMPR has many regulations that apply to the medical marijuana industry in Canada as a whole. Not all of those have implications for record keeping or to the method (software) used for that record keeping. (For links to the different websites that cover ACMP download our Seed-to-Sale Software Buyer’s Guide.)
How do I meet ACMPR compliance?
To be ACMPR compliant, your organization must have ways of meeting all of the regulations listed above.
Many of the regulations listed above apply to processes, so you’ll need to be utilizing best practices and documented standard operating procedures. You’ll also need to be able to prove to Health Canada that you have used best practices. That’s where seed-to-sale software comes in. Your cannabis business management software should provide you with the means to meet compliance when it comes to record keeping and reporting.
To support you in this process, a viable software vendor will supply you with a document that outlines exactly how the system they provide meets those requirements.
For a free demo to learn how AirMed meets these and other regulations, please email info @ airmed.ca.
What is seed-to-sale software and why do I need it?

Seed-to-sale software tracks plant production from reproduction through growth, harvesting, drying, packaging, sales and distribution. Health Canada mandates record keeping for every part of the process, and Canadian seed-to-sale software applications focus on compliance built on Health Canada’s ACMP (Access to Cannabis for Medical Purposes) regulations.
Record keeping is an essential part of Health Canada’s compliance regulations. From the advent of legal medical marijuana in Canada, legal producers of cannabis have been required to track every seed, rooted plant, gram of waste material, final dried product, as well as interactions with customers. Due to the sheer volume of information, an electronic record-keeping system is the only practical way to manage the process. The software industry has responded to this need by creating seed-to-sale management software systems designed to help producers track their operations and report to Health Canada to meet compliance.
As a Health Canada applicant, you must specify the name of the software system you plan to use for record keeping and provide a summary of how the software program meets Health Canada’s requirements. As a result, you will need to make at least preliminary decisions into seed-to-sale management software early on in your application process.
This might seem like one more challenge along your path to becoming a licensed producer, but in reality, seed-to-sale software is designed to help you be successful.
Seed-to-sale software platforms should provide the electronic record keeping required to not only for you to meet compliance, but also to help you be successful in your business. From greenhouse to warehouse to customer, your software system should help you at every stage of your operation. Functionality for genetics tracking, inventory management, quality control, shipping & receiving, point-of-sale ecommerce, compliance auditing & reporting, and customer relationship management can provide you with full business administration.
What is ACMPR and how does it affect me?
What does ACMPR cover?
For the cannabis industry in Canada, the most critical compliance implications are those covered by the Access to Cannabis for Medical Purposes Regulations. This legislation covers every aspect of marijuana production and sale. Overseen by Health Canada, these regulations must be adhered to at every stage of cannabis business from propagation through to sale. As of August 24, 2016, the Access to Cannabis for Medical Purposes Regulations (ACMPR) replaced the previous Marihuana for Medical Purposes Regulations (MMPR).
ACMPR is designed to provide an immediate solution for Canada to meet legal requirements. But Health Canada is continuously evaluating how a system of medical access to cannabis should function alongside the government’s commitment to legalize, strictly regulate and restrict access to cannabis. As a result, laws and regulations may change.
Currently, the legislation contains four parts.
Part 1 sets out a framework for commercial production by licensed producers responsible for the production and distribution of quality-controlled fresh or dried marijuana or cannabis oil or starting materials (i.e., seeds and plants) in secure and sanitary conditions.
Part 2 sets out provisions for individuals to produce a limited amount of cannabis for their own medical purposes or to designate someone to produce it for them.
Parts 3 and 4 include transitional provisions, consequential amendments to other regulations, and provisions repealing the MMPR.
All those wishing to produce cannabis in Canada must apply to Health Canada to become a licensed producer under the ACMPR legislation, and all licensed producers MUST comply with the ACMPR regulations.
What are the specific ACMPR regulations that affect software and record keeping?
The ACMPR has many regulations that apply to the medical marijuana industry in Canada as a whole. Not all of those have implications for record keeping or to the method (software) used for that record keeping. (For links to the different websites that cover ACMP see More Information at the end of this document.)
How do I meet ACMPR compliance?
To be ACMPR compliant, your organization must have ways of meeting all of the regulations listed above.
Many of the regulations listed above apply to processes, so you’ll need to be utilizing best practices and documented standard operating procedures. You’ll also need to be able to prove to Health Canada that you have used best practices. That’s where seed-to-sale software comes in. Your cannabis business management software should provide you with the means to meet compliance when it comes to record keeping and reporting.
To support you in this process, a viable software vendor will supply you with a document that outlines exactly how the system they provide meets those requirements.
For more information on seed-to-sale software, download our buyer’s guide, which answers to the following questions.
What does seed-to-sale software cost?
How do I choose one software platform over another?
What should I be looking for in the software?
How does the software fit into my business?
How is my data stored and secured?
What happens if regulations change?
Download the buyer’s guide here: How to purchase seed-to-sale software
For a free demo to learn how AirMed meets these and other regulations, please call 1-877-313-2442, use clikc the Request Demo button or email info@airmed.ca.