Selling internationally? You need GS1 and GTIN!

GTIN or Global Trade Item Number is a standard from the GS1 (Global Standards) organization. GTIN consists of unique codes that identify manufacturers and their products using barcodes. When scanned by an electronic reader, the GTIN barcode provides a code that is related to a specific manufacturer and a specific product from that manufacturer.
In North American, the UPC (Universal Product Code) is an existing form of the GTIN. In Europe, EAN-13 is the GTIN standard.
The GTIN system lets you and your products be identified across the globe. If you hope to sell in certain parts of the world, such as Europe, you will need to use GS1 standards and GTIN codes. Once you have signed up with your regional GS1 office, you will be issued a series of unique codes to use on your product packaging.
Fully supporting GS1, AirMed prints your GTIN barcodes directly from the database. Our master case processing lets you use multi-level barcoding for several layers of packaging or stock-keeping units (SKU). For example, one case could contain a dozen smaller cartons. Each of those cartons could contain a dozen retail-ready individual packages. All of those packaging layers can have its own barcode to meet the retail standards of the region where it will eventually be sold.
AirMed helps you meet your barcoding and packaging standards whether selling to a provincial warehouse or shipping internationally. AirMed even lets you apply pricing at the package or the master case SKU, and custom pricing can be set for specific SKUs by customer.
For more information on GS1 standards and GTIN barcoding, visit: https://gs1ca.org/
If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or click the Request Demo button at the top of the page to start the ball rolling.
Implementing AirMed in Your Facility

As a cloud-based platform, AirMed does not require expensive dedicated hardware to operate. Producers are free to use any computing device that supports a web browser. As a result, set up consists mainly of configuring the software to meet your needs. The rest of the time involves learning to use the system and getting your employees up and running.
How AirMed is implemented within your organization depends on the areas of the software you’ll be using. The first step in any software implementation is a needs assessment to help identify exactly how the software will be used. Understanding the capabilities of AirMed and how it can work with your processes is one of the keys to successful implementation. Your AirMed implementation specialist will work with you to perform an assessment of your needs to determine which areas of the software should be configured.
We provide an implementation guide and a production checklist that you can go through with your AirMed Implementation Specialist. Together, you’ll review the functionality in AirMed and determine which areas and functions are needed for your business operations. AirMed includes the ability to disable navigation menus for areas that you won’t be utilizing to provide a streamlined interface for your workers.
When you have completed your assessment and training, the next step is to configure your Live Production environment. After you’ve set up and tested your system, it’s time to train other users (your workers) to use AirMed.
Once users have usernames and passwords, they can access the training resources in the AirMed Learn environment. Workers can take additional training at a later date to develop skills for different areas of the system — in fact, anyone with access to AirMed can use the Learn environment at any time to practice new workflows or refresh their knowledge. When employees have completed training, you can give them access to the system, so they can get to work.
With AirMed you can implement only the functionality you need right now. For example, you can start by implementing the AirMed Grow module, then when you expand your operation, you add modules that meet your current needs for performing extractions, packaging for provincial sales, selling to medical patients or whatever your business entails.
Working with your implementation specialist will help you see all the benefits of using AirMed and ensure that you are getting the most from our software.

For more information on how AirMed helps specific types of businesses, visit our Customers page or our Frequently Asked Questions page.
Ready to learn more about AirMed? Click the Request Demo button or call 1-877-313-2442.
ISO, SCC and the Canadian Cannabis Industry

ISO, the International Organization for Standardization, is an independent, non-governmental international organization with a membership of 170 national standards bodies.
Through its members, ISO brings together experts to share knowledge and develop voluntary, consensus-based, market relevant International Standards that support innovation and provide solutions to global challenges.
In Canada, the ISO is represented by the Standards Council of Canada (SCC). A Crown corporation, SCC was established by an Act of Parliament in 1970 to foster and promote voluntary standardization in Canada. SCC is independent of government in its policies and operations, although it is financed partially by Parliamentary appropriation.
When Canada legalized cannabis in 2018, SCC set about creating standards for the new industry believing that standards are essential for an effectively regulated marijuana market. These standards were considered groundbreaking when published in October of 2022.
ISO IWA-37, “Safety, security and sustainability of cannabis facilities and operations” is available for purchase through ISO in three parts.
Part 1 (ISO IWA 37-1:2022) covers requirements for the safety of cannabis buildings, equipment and oil extraction operations. https://www.iso.org/standard/84023.html
Part 2 (ISO IWA 37-2:2022) covers requirements for the secure handling of cannabis and cannabis products. https://www.iso.org/standard/84024.html
Part 3 (ISO IWA 37-3:2022) covers good production practices (GPP). https://www.iso.org/standard/84025.html
Taken as a whole, these documents provide invaluable direction to legislative bodies and emerging companies and help to create a safe, legal market for adults who use cannabis.
For more information from SCC visit: https://www.scc.ca/en/news-events/news/2022/new-guidance-from-iso-international-workshop-safe-cannabis-production
For more information from ISO visit: https://www.iso.org/en/contents/news/2022/10/standards_safe_legal_cannabis.html
To learn more about how AirMed helps you meet these standards, visit our Compliance page.
If you’d like to learn about our quality management and GPP offerings or discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms.
QMS-GPP Planning According to ISO:9000
Introduction
Quality management is the practice of ensuring consistency in products and services throughout your organization. A Quality Management System (QMS) helps your cannabis business provide customers with the best you can offer while mitigating risks using tools that support Health Canada’s Good Production Practices.
A Quality Management System-Good Production Practices Plan provides an overview of the quality management system that an organization has in place. Although there are many standards in the world, ISO:9000 is one of the most respected. And according to ISO:9000 standards, the plan must contain the following.
Quality Policy (ISO:9000 clause 5.2): A quality statement can be derived from a mission statement and/or vision statement, but should explain the organization’s commitment to quality
Quality Objectives (ISO:9000 clause 6.2): These can be the organization’s objectives from a business plan, again, as long as they contain a commitment to quality
Criteria for Evaluation and Selection of Suppliers: As quality management and good production practices are often dependent on supplies and equipment that come from other organizations, organizations need to have a criteria in place for evaluating their suppliers to ensure that they select suppliers that meet QMS and GPP standards
Scope of the QMS: This is a list of all SOPs with brief descriptions/purposes
Quality Product Statement
ISO:9000 requires your organization’s quality policy to be appropriate to your organization’s strategic direction and operational direction (context).
Your organization must understand and identify all the influences that affect its business and ensure that the strategy and direction takes quality into consideration. Your organization will need to review its current quality policy regularly to ensure that any changes in context, interested parties or other requirements are reflected, and to determine whether your organization’s objectives are affected. (ISO 9001:2015 – 6.2.1a.)
Following is an example.
Company ABC produces cannabis products for distribution in Canada according to the regulations in the Cannabis Act. The Company has developed its production system through experience and its aim is to achieve a high standard of production and products to its customers.
It is the policy of Company ABC to provide the customer with goods to the agreed requirement in accordance with the details and price.
The Directors, Management and Staff are responsible for Quality Control through the Quality Management System seeking improvement by constant review, with suppliers and sub-contractors being encouraged to co-operate. The Company is committed to achieving customer satisfaction by the use of quality procedures which will be operated to meet or exceed the requirements of [the Cannabis Act and/or ISO 9001 or other quality system].
Quality Objectives
The quality objectives should act as a driver for continual improvement. To meet quality standards, your organization will be required to ensure that you continually improve products and services to meet customer requirements and to measure effectiveness of the processes responsible.
Following is an example.
Company ABC strives to be the best provider of cannabis products in Canada. Through the use of this guiding principle, everyone in Company ABC is accountable for fully satisfying our customers and authorities by meeting or exceeding their needs and expectations with best-in-class production practices. Our goal is 100% customer satisfaction and compliance 100% of the time.
Our Quality Policy is defined and strongly driven by the following objectives:
1. Meet all compliance requirements for all levels of governments and regulatory agencies
2. Build a mutually profitable relationship with our customers, ensuring their long-term success, through the understanding of their needs and the needs of their customers as well
3. Achieve our commitments for quality, cost, and schedule
4. Use of best preventive practices at all levels and ensure reliable risk management
5. Drive continual improvement and innovation based upon efficient business processes, well-defined measurements, best practices, and customer surveys
6. Develop staff competencies, creativity, empowerment and accountability through appropriate development programs and show strong management involvement and commitment
Evaluation and Selection of Suppliers
Supplier evaluation is a system for recording and ranking the performance of a supplier in terms of a variety of criteria and is a must in ISO:9000. A process of vendor rating is essential to effective purchasing. While there is no one right system for supplier evaluation and selection process, the overall objective is to reduce risk and maximize overall value to the purchaser.
Criteria
There are eight common supplier selection criteria:
1. Cost
2. Quality & Safety
3. Delivery
4. Service
5. Social Responsibility
6. Convenience/Simplicity
7. Risk
8. Agility
In the cannabis industry, you should also add a commitment to meeting compliance and/or helping their customers meet compliance.
Methods
There are many other methods of evaluation, and the organization should determine which is the best for its use.
Categorical systems typically use excellent, good, average, poor and so on.
Weighted systems rate on a scale from 1 to 10 or out of 100.
Hierarchical systems give values in relation to each item’s importance. The most important item is given the highest value.
Conclusion
Of course, a quality management system and good production practices plan is only as good as the processes that support it. Creating standard operating procedures and ensuring that all personnel follow them will give you the best chance of success.
For more information about ISO:9000 visit: ISO – ISO 9000 family — Quality management
For information on how AirMed helps you meet compliance, visit our Compliance page.
If you’d like to learn about our quality management and GPP offerings or discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms.
AirMed and FDA CFR 21

We are sometimes asked if AirMed meets FDA standards. First, please be aware that the FDA is a department of the US government. The specific portion of FDA regulations relevant to software such as AirMed is part CFR 21. As it is an American standard, Health Canada does not require CFR Part 21 compliance as part of the Cannabis Act Regulations. And while AirMed was designed for the Canadian cannabis industry and to comply with Health Canada regulations, AirMed does conform to CFR Part 21 with respect to electronic record keeping, audit trails, and electronic signatures.
Many agencies throughout the world are responsible for issuing and enforcing regulations that affect businesses. The regulations affecting software such as AirMed are typically those related to records management compliance. And while there are many regulatory agencies involved in records management, for the most part the regulations themselves are similar from country to country and agency to agency. The purpose of them, in general, is to ensure the security, confidentiality and authentication of electronic records.
The U.S. FDA regulates food, drugs, medical devices, biologics, animal feed and drugs, cosmetics and radiation-emitting products such as cell phones for the U.S.A. The FDA’s rules for manufacturing and distribution are designed to protect consumers and promote public health. In the U.S. Code of Federal Regulations (CFR), Title 21 deals with Food & Drugs. Until recently, the regulations in this title required paper records with handwritten signatures.
Back in 1997, part 11 of 21 CFR was enacted to cover the use of electronic records and electronic signatures. Commonly known as 21 CFR 11, this part defines the criteria “under which the agency considers electronic records, electronic signatures, and handwritten signatures executed to electronic records to be trustworthy, reliable, and generally equivalent to paper records and handwritten signatures executed on paper.”
Essentially, the concerns about using electronic records are that records may be lost in a system crash, the data may become corrupt or modifications may be made without proper authorization. In addition, since printed documents with hand-written signatures are recognized as legally binding on the signators, the agencies are looking for ways to make electronic records similarly binding on their owners. The regulations have been proposed to ensure that whenever an organization replaces printed documents with electronic data, there are checks and balances in place to ensure integrity of the electronic records so that they can be legally equivalent to printed records.
AirMed has a range of features that satisfy standards for security, authentication, validation and auditing as outlined in 21 CFR 11 and other regulations.
For more detailed information visit: Code of Federal Regulations (CFR) | FDA
For more information on how AirMed helps you meet compliance, visit our Compliance page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
New reports in AirMed keep pace with marketplace

AirMed has the most comprehensive reporting in any cannabis management system. To ensure that our reporting keeps pace with changes in regulations and the cannabis marketplace, we regularly update our reporting system and add new reports.
Industry analysts and market researchers agree that edibles are the future of cannabis as consumer preferences shift from away from smoking.
Seattle-based data-analytics firm Headset examined sales from the US and Canada and reported “…overall edibles sales grew by more than 20%…”
According to a different report titled Cannabis Edible Products Market Report, “The aversion to smoking is one of the main factors that has led to the significant growth of cannabis-infused edibles over the past years.”
MJ Biz Daily reported that “The outlook for edibles is considered bullish among industry executives… Industry executives said consumers around 30-45 years old especially seem to be entering the cannabis market via edibles.”
To support the addition of edibles and topicals functionality in AirMed earlier this year, we added new CTLS data reporting. We also updated the existing CTLS Full Export function to include the totals from the main CTLS reports.
The release of edibles and topicals functionality in tandem with supporting CTLS reporting lets users manage entire edible and topical inventory data in AirMed and also export data into a pre-populated CTLS monthly report using the CTLS Full Export function. This vastly improves the inventory tracking capabilities for edibles and topicals and can save users valuable time in monthly reporting.
AirMed is working hard to support consumer trends so cannabis producers and processors can meet the needs of the marketplace.
The “Cannabis Edible Products Market Report” can be found at: https://www.researchandmarkets.com/reports/5529328/cannabis-infused-edible-products-market-growth
The Headset “Cannabis Product Trends” report can be found at: https://www.headset.io/industry-reports/the-most-popular-cannabis-product-trends-in-the-us-canada
Read more from MJ Biz Daily on the edibles market here: https://mjbizdaily.com/led-by-gummies-edibles-keep-pace-with-growth-of-overall-us-marijuana-market/
To learn more about AirMed reporting visit: https://airmedcloud.com/software/#report
For a demo of AirMed’s reporting features, click the Request Demo button at the top of the page or use the contact form in the footer. You can also call us directly at 1-877-313-2442.
Quality Management and AirMed

Constantly innovating to help our clients, we have a host of features in development for our seed-to-sale software. One of the most significant improvements currently in development for AirMed is built-in Quality Management.
A Quality Management System (QMS) can help your organization mitigate regulatory risk by providing the tools you need to meet the requirements and standards for Health Canada’s Good Production Practices. But risk reduction is not the only benefit of QMS. You can also improve efficiency, provide consistent control over processes, and much more. As a result, we’re building a range of QMS features into AirMed including the following.
- Escalation and Tracking: Escalation and tracking will be available for use in any area of the facility such as plant growth incidents, extraction incidents, order and fulfillment incidents.
- Vendor Approval Process: A multi-level approval process will be supported for new vendors who supply products.
- Enhanced Received Product Management: New features are being added for received product quarantine, testing and release for use. The new functionality will support having certain received products undergo QA to ensure they match specifications. QA results can be uploaded to support this workflow.
- SOP Management: Standard Operating Procedures (SOP) are critical to QMS, and we will be adding SOP management for current and historical SOPs with auto-versioning to track changes in procedures.
- Electronic Signatures: Electronic signatures will be available for workflows such as destruction, sanitation, and processes that need approval from a responsible person in charge.
- Case Management for Plant Issues: With new support for both SMS and MMS notifications, a grow tech concerned about a plant will be able to send a photo to the head grower for instant review.
We’re working hard to meet shifting customer needs and market demands in the Canadian cannabis industry, turning your feedback into important product advancements. AirMed seed-to-sale business solutions go beyond compliance to help licensed producers cultivate success.
If you’d like to read about Canada’s Good Production Practices for Cannabis, visit https://www.canada.ca/en/health-canada/services/cannabis-regulations-licensed-producers/good-production-practices-guide.html
For more information about AirMed visit our software page.
If you have specific questions or would like a free demo of AirMed, please contact an AirMed sales representative by filling out one of our contact forms or calling 1-877-313-2442.
Software Validation for Cannabis Processors

One of the challenges for cannabis processers is that regulations require them to validate their extraction practices. In many cases, those practices involve the use of software. Therefore, any software used in relation to production must be validated along with the practices.
As tracking production reliability is an important part of meeting compliance, most tracking involves the use of software.
Software vendors are responsible for ensuring the software they sell works as described. But, validating that software is the responsibility of the processor. Why? Because every organization operates differently, and validation must be done in relation to actual production practices.
If you are using AirMed as your system-of-record, you will need to validate our software as it is used in your facility.
AirMed helps you through the validation process by providing test scripts that can be used to validate each task in the workflows that relate to extraction processes. These test scripts show what the software was designed to do, how the software should be used for that specific task and what the end result should be.
We can provide you with a validation report outlining how we meet software vendor compliance. Our validation report details how our software complies with security, privacy and accessibility requirements. AirMed software has been designed to not only meet compliance with Canada’s Cannabis Act but also to support and comply with Federal and Provincial privacy legislation and regulations (PIPEDA, PIPA, PHIPA, PHIA). AirMed also meets the standards outlined in the US FDA 21 CFR Part 11, which many other specifications are based on.
Our internal procedures have been through an external vendor audit for the pharmaceutical industry and passed with no observations.
If you’d like to learn more about good production practices for cannabis in Canada, the Government of Canada has published GPP guidance information here: https://www.canada.ca/en/health-canada/services/cannabis-regulations-licensed-producers/good-production-practices-guide/guidance-document.html
For more information on how AirMed helps you meet compliance, visit our Compliance page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
Role-based Management in AirMed
Role-based management in AirMed helps control employee access to the software system.
Actions within AirMed are linked to roles. When you assign a role to an employee, you allow access to those actions.
Employees can be assigned to the roles that correspond to the requirements of their jobs. Employees not assigned to specific roles are prevented from accessing the associated workflows.
For example, if a staff member is new and working in cultivation, you can create a role that only provides access to certain actions in the Source Material, Batch, and Lot Details screens. All other action items will not be visible. This lets you restrict access to parts of the system, based on a user’s job function within your organization.
AirMed has several roles defined by default, but you can create as many roles as required. You can also edit or rename existing roles to match your organization’s workflows. Within each role you can specify which actions are associated.
For more information about how roles can help you manage employee access, contact us today by using one of the contact forms or by calling 1-877-313-2442.
For more information on how AirMed helps you business, visit our Software page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
AirMed still 100% Canadian owned

As US corporations buy up seed-to-sale software companies in Canada, AirMed is still 100% Canadian owned.
AirMed was created in 2014 to help Canadian licensed producers meet compliance at all levels of government. Continuously innovating since then, we believe that building a culture of quality is an important part of our customers’ success. This commitment to quality is why a growing list of cultivators, nurseries, processors, manufacturers, and dispensaries use AirMed.
We rely on industry requirements and customer feedback to drive AirMed development, rather than investor pressure. We encourage our users to tell us what matters most to them in a cannabis management system. This feedback helps guide us in adapting AirMed to improve efficiency, productivity and user experience.
Our goal is to provide a responsive solution that not only meets customer needs but anticipates them, regardless of the size or focus of your cannabis business. That has been our driving force for nearly eight years.
Contact AirMed for a demo today to see what homegrown can do for you. Call us at (877) 313-2442 or use the contact form in the footer of this page.
For more information about AirMed, visit our About page or our Frequently Asked Questions page. If you’d like to have a conversation about AirMed, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
Support for GS-1 Barcoding Standards in AirMed
AirMed software offers full functionality for multi-level packaging with layered barcoding that meets GS-1 barcode standards. This is the standard required to sell to provincial government distributors.
Licensed producers who use AirMed can assemble retail-ready individual product packages using a ‘master case’ system and identify and track products at each packaging level.
AirMed software offers full SKU and barcode management that supports GS-1 barcoding standards. Pricing can be set at the package or master case SKU, and custom pricing can be set for specific SKUs by customer. The software offers features for creating and processing the orders as well as fulfilling and shipping them to provincial agencies.
Our master case processing lets you use layered barcoding for individual containers through to cases of multiple containers. The AirMed track-and-trace system allows retail packages to be traced to the lot or batch and ultimately back to the original genetic material. And AirMed has a comprehensive multi-step recall process that completely automates recalls if they are ever needed.
To schedule a live demonstration of AirMed Cloud software, call us at 1-877- 313-2442 or click the Request Demo button at the top of the screen.
For more information on how AirMed helps your business, visit our Software page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
Manage Plants Individually or by Group in AirMed
AirMed gives you the flexibility to choose whether to manage plants individually or in groups.
Individually managed plants allow for each plant to be tracked independently using its unique barcode. This method of tracking the plants offers full precision by providing detailed information about the individual plant and letting you perform actions on each plant separately. Plant actions are recorded by scanning the barcode associated with the plant using a tag or container label.
Individual management is usually reserved for small batches or for mother plants where tracking of the individual plant is beneficial. Group management is recommended for larger crops intended for harvest as the tracking is simplified.
Group-managed plants are recorded by counts and do not need to be identified by a unique barcode, so there is no requirement to print off labels for each plant. When selecting plants to perform an action at the batch level, a count of plants is recorded for either a table or a room.
Plants are propagated as part of a batch, and records are maintained through each grow phase such as drying or harvesting at the batch level. The production process ends with the creation of a lot, which is linked directly to the batch. Post-production, including bulk storage or packages, is also tracked by the batch.
By default, plants are individually managed until you make the decision to covert them to group management. AirMed lets you choose which method is the most appropriate and efficient while still maintaining accurate records.
To find out more about AirMed features like group management, call us today at 1-877-313-2442 or use one of the contact forms to request a free demo.
CTLS Reporting in AirMed
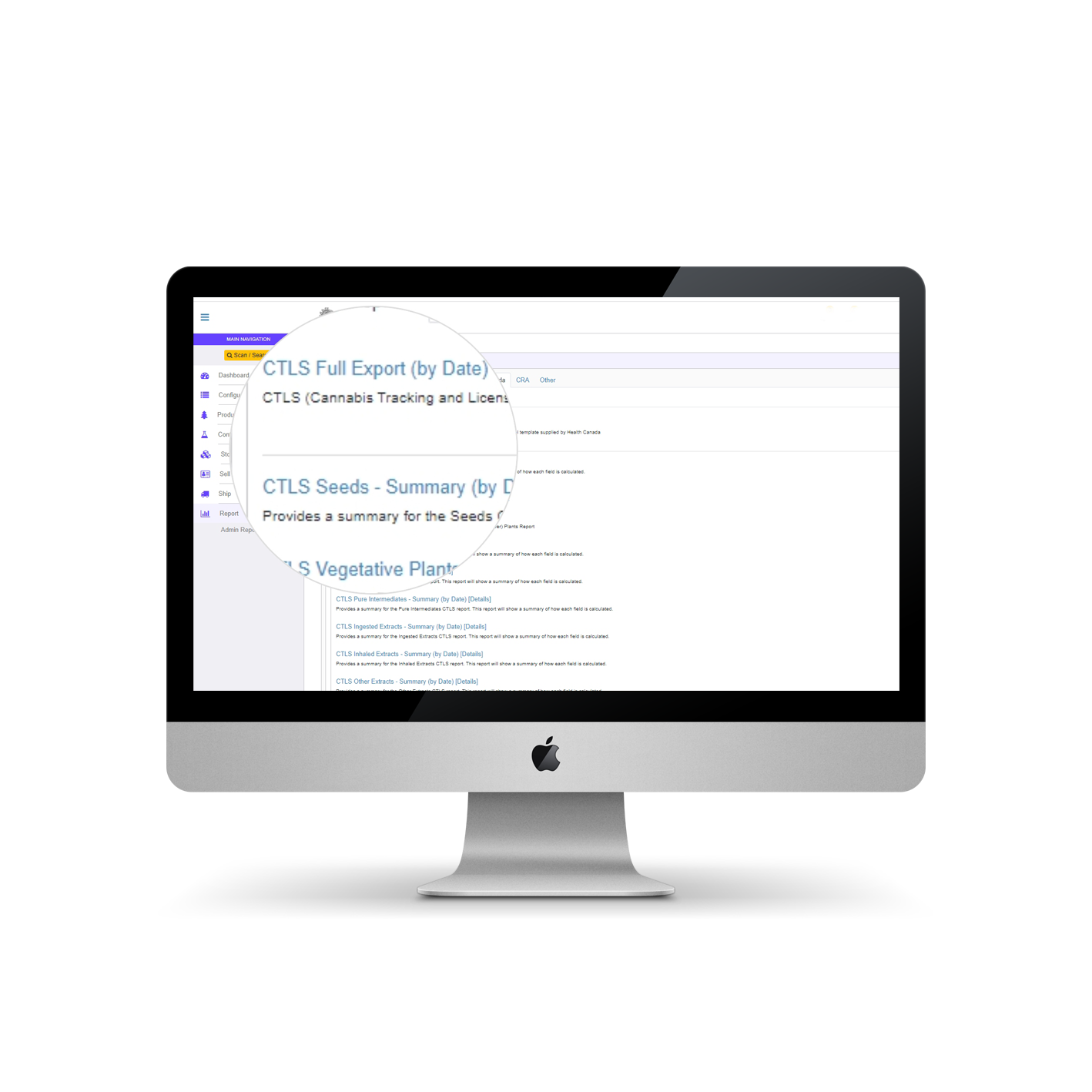
Always innovating to help our customers meet the regulatory challenges they face daily, AirMed offers a new automated system for CTLS reporting.
As mandated by the Cannabis Act, Health Canada requires that licensees submit a Cannabis Tracking and Licensing System (CTLS) report every month for every licensed site. This reporting applies to cultivators and processors (including micro, standard or nursery), as well as sellers of medical cannabis and provincial or territorial authorities, distributors and retailers.
Full Export
Using our new full export process, thousands of reported fields tracked by AirMed will automatically populate the standard spreadsheet template provided by Health Canada. Not only is the time required to complete monthly reports reduced, but the potential for human error often encountered in manual data entry is eliminated.
Summary Reports
AirMed also includes a suite of CTLS summary reports offering breakdowns of key fields with direct links to detailed reports. You can identify the values used to calculate a final total and also find the exact inventory records used to calculate those values, all from one page. Inventory validation has never been easier.
For more information or for a free demonstration of our automated CTLS reporting, please call 1-877-313-2442 or use the contact form linked at the top of the page. In the meantime, visit our Software page.
Quality Assurance in AirMed

AirMed provides licensed producers with a complete framework to establish and validate a quality assurance system.
The AirMed QA system is based on pre-defined North American and European pharmacopeia.
The system tracks testing results for the samples taken at any stage of production and enables shipping of samples to third-party QA labs. Results and certificate of analysis documents are logged and used to determine if the material the sample was derived from is acceptable for sale. Test results are verified by an authorized QA person and a passed or failed status is assigned to each data point. The QA results are maintained in the system and can be referenced at any point to review the values.
From reviewing non-conformance to documenting and communicating findings, AirMed helps you maintain your desired level of quality. Tracking attention to detail for every process gives you total confidence in your product.
For more information about quality assurance in AirMed, visit our Software page. You can also schedule a live preview of the software by clicking the Request Demo button at the top of the screen. Or give us a call at 1-877-313-2442.
Reporting & Auditing in AirMed

AirMed software is specifically designed to meet or exceed Cannabis Act regulations with full audit support capabilities and comprehensive reporting. AirMed regularly passes Health Canada inspections. For more information visit our Compliance page.
AirMed is designed to support and comply with federal, provincial and local legislation. In fact, AirMed meets the USA’s FDA 21 CFR Part 11 regulations. Considered to be some of the most comprehensive electronic record keeping specifications in the world, they are the basis for the standards set by many countries.
AirMed pulls all the required information for all government reporting, whether monthly, quarterly, or annually. Reports are built in for Cannabis Licensing & Tracking System (CTLS) and Canada Revenue Agency (CRA). Supporting sub-reports provide the necessary validation detail. Export data to other systems or print reports to retain the required information outside of AirMed as archived printed or electronic records.
Every action/workflow in AirMed is auditable, and built-in audit reports & forms provide complete product traceability. Inventory, recall, destruction and other reports can be generated as needed. Authorized site auditors can be given access to perform inspections of records. AirMed retains all data indefinitely and lets you export or print reports to be held for required retention periods for audit purposes.
For more information about AirMed’s reporting and auditing features, schedule a free demo by clicking the Request Demo button at the top of the page or by using the contact form in the footer. You can also call us directly at 1-877-313-2442.
How Secure is AirMed Software?

AirMed seed-to-sale software is a Cloud-based platform hosted in a third-party, Tier 3, SSAE 18 SOC1/SOC2 compliant, hardened data center in Canada, and all data remains in Canada. The same data center is used by Canadian banks and government agencies to secure their web-based applications. All data in AirMed is protected by fully managed and monitored enterprise firewalls and intrusion detection/prevention systems.
All communication between the browser and the server is encrypted using SSL. Sensitive data is additionally encrypted while at rest in the database. When a barcode is scanned, the resulting code is input into a field within the web browser. This data is then encrypted and sent to the server for processing.
Administrators can configure access based on an employee security profile so that individual workers only have access to the functions and data that relates to their job responsibilities. Electronic signatures and multi-factor authentication ensure only authorized users can access information and complete tasks. Full audit trails for every record and every action in the system allows administrators to review changes anywhere at any time.
All data is backed-up both locally in the data centre for near-line recovery and to a secure offsite location in Canada for disaster recovery purposes.
To learn more about AirMed’s privacy and security, contact us today by using the contact form or by clicking the Request Demo button at the top of the screen. You can also give us a call at 1-877-313-2442.
New AirMed version lays groundwork for October 17 Cannabis Act changes

A new version of AirMed seed-to-sale software was released October 16, 2019. This is a substantial build that lays the groundwork for the October 17 changes to the Cannabis Act and CTLS reporting.
The new version offers significant updates to the Received Products section of the cloud-based application including the ability to receive into the facility any type of product (edibles are coming soon) either bulk or packaged. The system now allows product to be transferred to a processor and packaged up, then the packaged material can be received back into the facility.
For received products, users now have the ability to add items to an existing lot, back-date inventory adds, and update finished/unfinished designation for new Health Canada regulations. We’ve also added an extraction material type category for new Cannabis Tracking and Licensing System (CTLS) reporting requirements, plus the ability to move all plants in batch and print a timeline report for batch details.
If you are a current AirMed customer, please explore the new features in your sandbox service, and let us know if you have any questions by contacting your customer service representative.
To learn more about the Cannabis Act in Canada visit: What you need to know about cannabis – Canada.ca

For more information on how AirMed helps your cannabis business, visit our Software page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.
AirMed Ecosystem: Industrial tablets for your seed-to-sale business

AirMed recommends industrial tablets for use in your cannabis business. These ruggedized hand-held devices are waterproof and dust proof and can withstand a drop from five feet on a concrete floor. They have a built-in barcode scanner and camera and come loaded with Windows 10. The tablets feature a docking station with USB ports for printers, scales, keyboard, mouse and other peripherals.
When configured with AirMed Cloud software and attached to a printer and scale, tablets function as a self-contained workstation to carry out all process-related tasks. They can be integrated with barcode software to capture data throughout your operations. A tablet can serve as the computer for a specific work area such as a nursery or mother room, grow or drying room, laboratory, warehouse or loading dock.
These ruggedized industrial tablets are sold directly through AirMed Canada Systems Inc. and work with Windows applications. If you purchase a tablet through AirMed, it can be pre-configured with AirMed Cloud software as well as Winwedge software for barcode scanning.
These tablets are part of our new AirMed Ecosystem, which integrates AirMed with couriers, printers, scanners, ecommerce software and more. Our new AirMed Ecosystem lets you purchase hardware and software from us in a turn-key solution for your cannabis business. For more information visit our Partners page.
Each product selected for the AirMed Ecosystem is carefully chosen based on its ability to provide value, lower production costs and increase competitiveness for AirMed customers. You create the package that’s right for you from pre-configured hardware and software.
AirMed offers the tools you need in the office, the greenhouse and on the loading dock. Our Ecosystem lets you purchase hardware and software in a turn-key
seed-to-sale management solution.
To learn more about our Ecosystem program, visit: AirMed Ecosystem
To learn more about Winwedge software visit: TalTech
Introducing the AirMed Ecosystem

To help our clients cultivate success, AirMed is developing strategic partnerships with leading vendors of products and services in the Canadian cannabis industry. We’ve partnered with the best in the industry including compliance consultants, hardware manufacturers, telecommunications and delivery services.
The AirMed Ecosystem provides a turn-key solution with integrated hardware and software to help producers become more productive and profitable. From pathogen detection and environmental analytics through barcoding and label printing to ecommerce and delivery, the AirMed Ecosystem provides the best the industry has to offer for your cannabis business.
If you are a licensed producer or cultivator, visit our partnering page to find out more about what we offer and the awesome companies we’ve partnered with: AirMed Ecosystem
If you provide a complementary product or service in the cannabis industry and would like to collaborate with AirMed, click here for a form to request a conversation with a partnering representative: Partner Form
And if you’d like a product demo of AirMed software, call (877) 313-2442 or email info @ airmed.ca.
AirMed supports wholesaling to provincial distributors

AirMed Cloud seed-to-sale software supports wholesale selling and shipping from licensed producers to other LPs and from LPs to provincial distribution centres.
As a cannabis management system, our software offers full functionality for multi-level packaging with layered barcoding that meets GS-1 barcode standards. LPs using AirMed can assemble retail-ready individual product packages using a ‘master case’ system and identify and track products at each packaging level.
“Our master case processing lets you use layered barcoding for individual containers through to cases of multiple containers,” noted Justin Hearn, President and CEO of AirMed Canada Systems Inc. “AirMed’s track-and-trace system allows retail packages to be traced to the lot or batch and ultimately back to the original genetic material. And AirMed’s comprehensive multi-step recall process completely automates recalls if they are ever needed.”

AirMed software offers full SKU and barcode management that supports GS-1 barcoding standards. Pricing can be set at the package or master case SKU, and custom pricing can be set for specific SKUs by customer. The software offers features for creating and processing the orders as well as fulfilling and shipping them to provincial agencies.

“We’re proud to be supporting our customers with full master case inventory control, GS1 integration, comprehensive wholesale order management, efficient fulfillment and tracking, and compliance reporting,” said Hearn. “AirMed’s wholesale distribution module is helping LPs produce, prepare, sell and ship hundreds of thousands of products to the recreational market right across Canada.”
For more information on how AirMed helps your business, visit our Software page or our Frequently Asked Questions page. If you’d like to discuss your specific needs, please give us a call at 1-877-313-2442 or use one of the contact forms to start the ball rolling.






